正在加载图片...
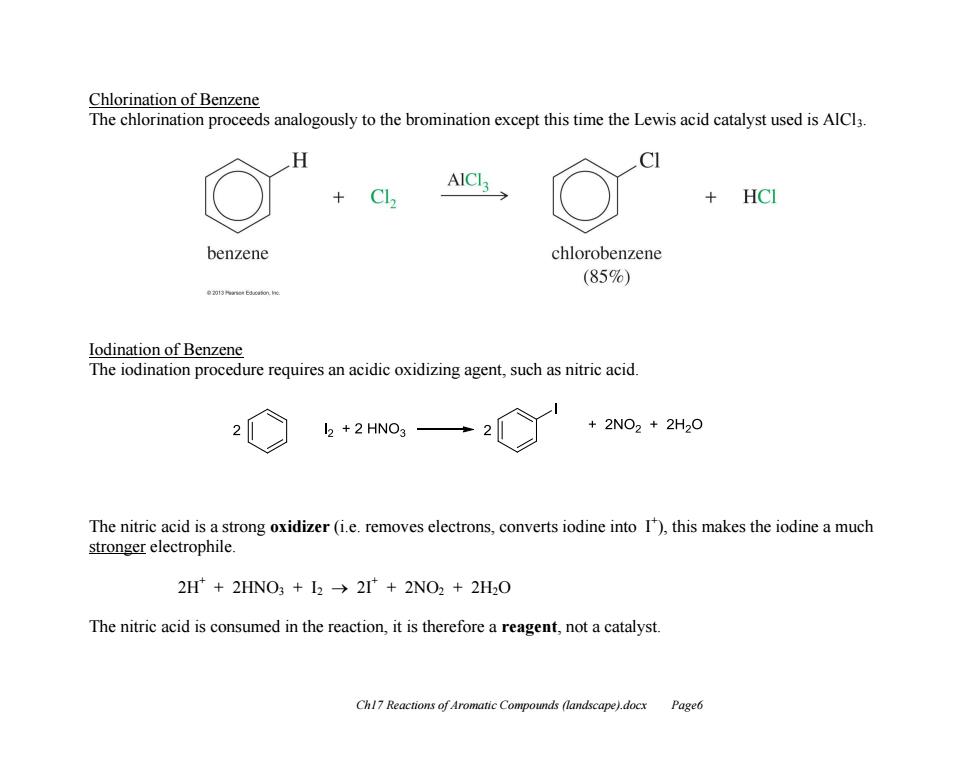
Chlorination of Benzene The chlorination proceeds analogously to the bromination except this time the Lewis acid catalyst used is AlCl3 H AICI HCI benzene chlorobenzene (85%) e23nne6运am.e lodination of Benzene The iodination procedure requires an acidic oxidizing agent,such as nitric acid l2 +2 HNO3 +2NO2+2H20 The nitric acid is a strong oxidizer(i.e.removes electrons,converts iodine into 1),this makes the iodine a much stronger electrophile. 2H+2HNO3 I2 2I+2NO2 +2H2O The nitric acid is consumed in the reaction,it is therefore a reagent,not a catalyst Chl7 Reactions of Aromatic Compounds (landscape).docx Page6 Ch17 Reactions of Aromatic Compounds (landscape).docx Page6 Chlorination of Benzene The chlorination proceeds analogously to the bromination except this time the Lewis acid catalyst used is AlCl3. Iodination of Benzene The iodination procedure requires an acidic oxidizing agent, such as nitric acid. The nitric acid is a strong oxidizer (i.e. removes electrons, converts iodine into I+ ), this makes the iodine a much stronger electrophile. 2H+ + 2HNO3 + I2 2I+ + 2NO2 + 2H2O The nitric acid is consumed in the reaction, it is therefore a reagent, not a catalyst