正在加载图片...
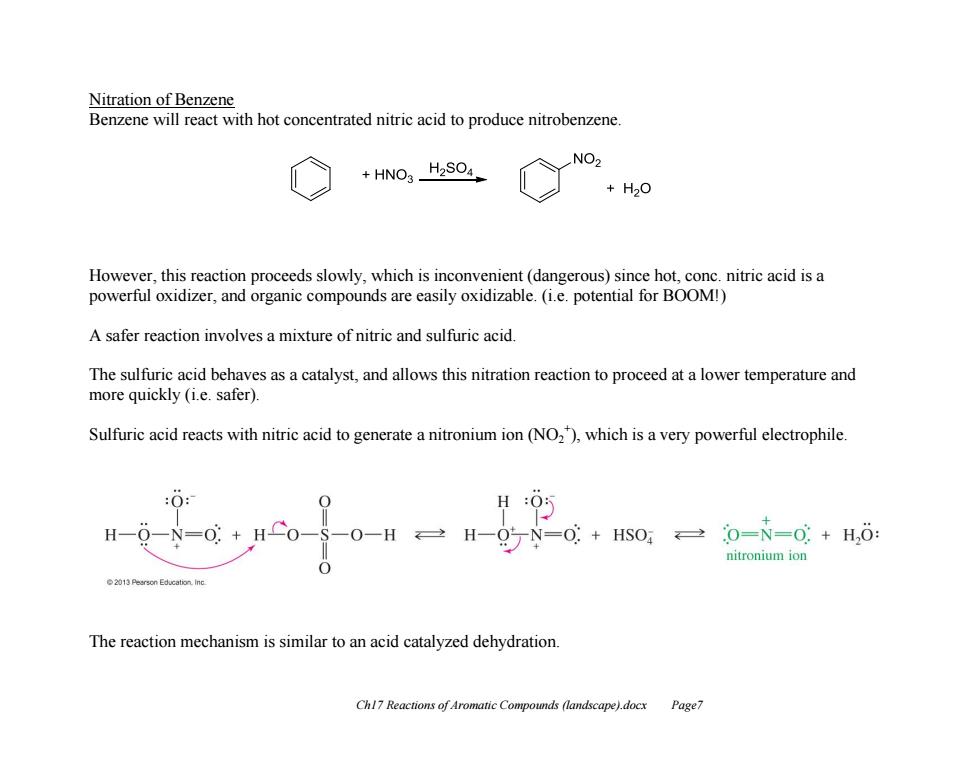
Nitration of Benzene Benzene will react with hot concentrated nitric acid to produce nitrobenzene. HNO3_H2SO4 NO2 +H20 However,this reaction proceeds slowly,which is inconvenient(dangerous)since hot,conc.nitric acid is a powerful oxidizer,and organic compounds are easily oxidizable.(i.e.potential for BOOM!) A safer reaction involves a mixture of nitric and sulfuric acid. The sulfuric acid behaves as a catalyst,and allows this nitration reaction to proceed at a lower temperature and more quickly (i.e.safer). Sulfuric acid reacts with nitric acid to generate a nitronium ion(NO2),which is a very powerful electrophile :O: H H -N=0: 0+HS0,0=N=0+H,: nitronium ion 0 The reaction mechanism is similar to an acid catalyzed dehydration Chl7 Reactions of Aromatic Compounds (landscape).docx Page7Ch17 Reactions of Aromatic Compounds (landscape).docx Page7 Nitration of Benzene Benzene will react with hot concentrated nitric acid to produce nitrobenzene. However, this reaction proceeds slowly, which is inconvenient (dangerous) since hot, conc. nitric acid is a powerful oxidizer, and organic compounds are easily oxidizable. (i.e. potential for BOOM!) A safer reaction involves a mixture of nitric and sulfuric acid. The sulfuric acid behaves as a catalyst, and allows this nitration reaction to proceed at a lower temperature and more quickly (i.e. safer). Sulfuric acid reacts with nitric acid to generate a nitronium ion (NO2 + ), which is a very powerful electrophile. The reaction mechanism is similar to an acid catalyzed dehydration