正在加载图片...
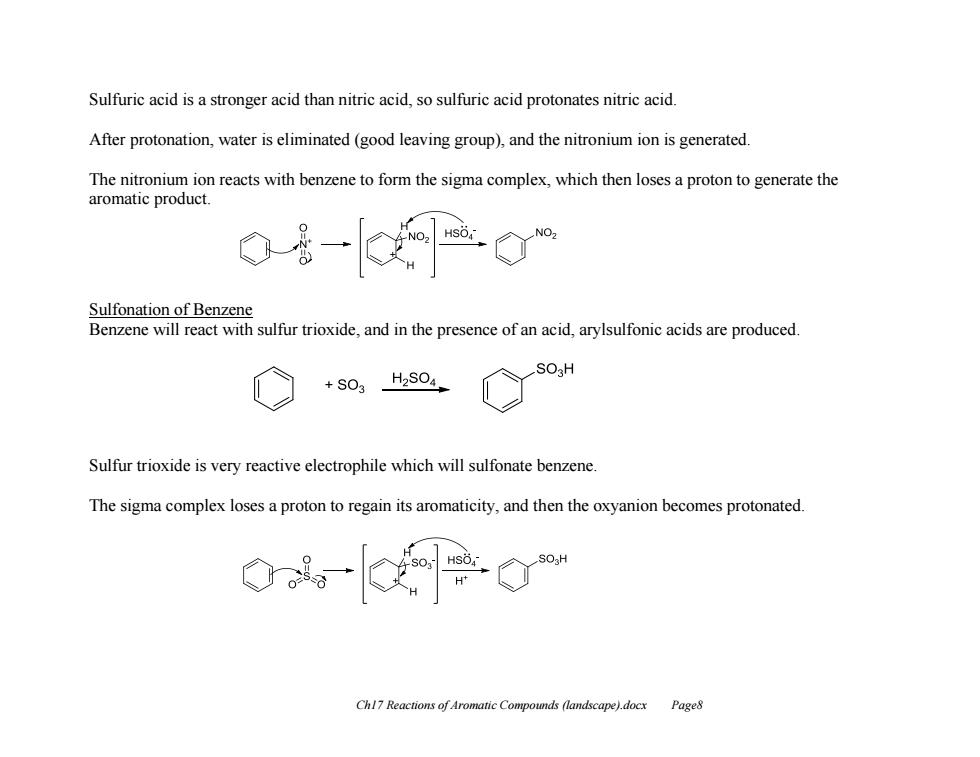
Sulfuric acid is a stronger acid than nitric acid,so sulfuric acid protonates nitric acid. After protonation,water is eliminated (good leaving group),and the nitronium ion is generated The nitronium ion reacts with benzene to form the sigma complex,which then loses a proton to generate the aromatic product. Sulfonation of Benzene Benzene will react with sulfur trioxide,and in the presence of an acid,arylsulfonic acids are produced. +S03 H2S04 Sulfur trioxide is very reactive electrophile which will sulfonate benzene. The sigma complex loses a proton to regain its aromaticity,and then the oxyanion becomes protonated. Chl7 Reactions of Aromatic Compounds (landscape).docx Page8 Ch17 Reactions of Aromatic Compounds (landscape).docx Page8 Sulfuric acid is a stronger acid than nitric acid, so sulfuric acid protonates nitric acid. After protonation, water is eliminated (good leaving group), and the nitronium ion is generated. The nitronium ion reacts with benzene to form the sigma complex, which then loses a proton to generate the aromatic product. Sulfonation of Benzene Benzene will react with sulfur trioxide, and in the presence of an acid, arylsulfonic acids are produced. Sulfur trioxide is very reactive electrophile which will sulfonate benzene. The sigma complex loses a proton to regain its aromaticity, and then the oxyanion becomes protonated