正在加载图片...
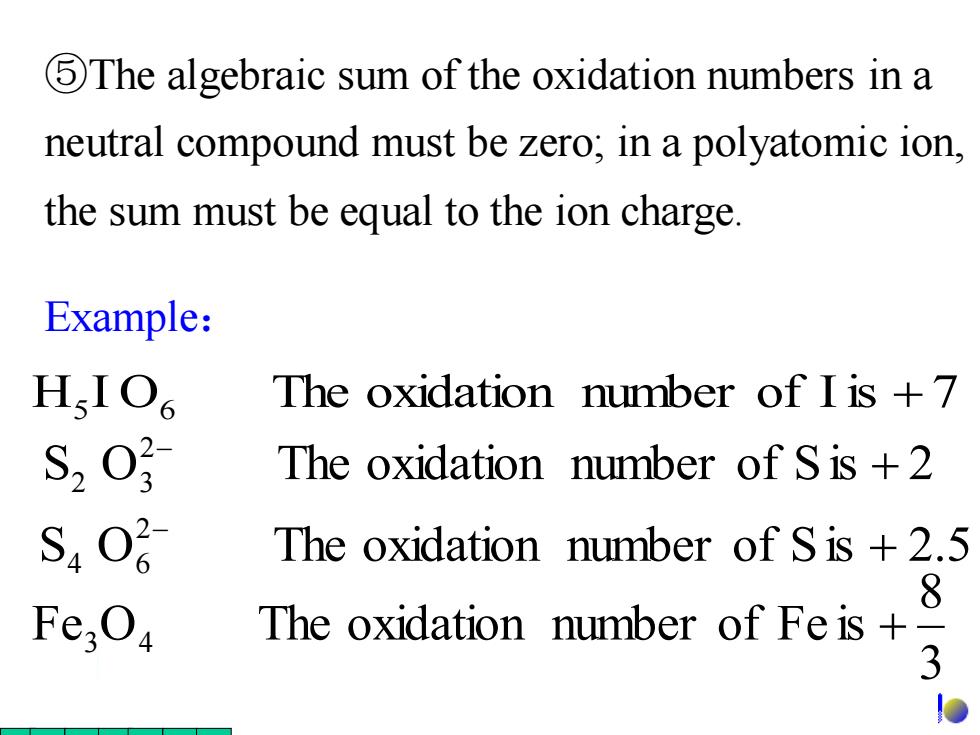
5The algebraic sum of the oxidation numbers in a neutral compound must be zero;in a polyatomic ion, the sum must be equal to the ion charge Example: HIO The oxidation number of I is +7 The oxidation number of S is +2 S40 The oxidation number of S is +2.5 8 Fe;O The oxidation number of Fe is+ 3 DExample: ⑤The algebraic sum of the oxidation numbers in a neutral compound must be zero; in a polyatomic ion, the sum must be equal to the ion charge. H5 I O6 The oxidation number of Iis + 7 3 8 Fe O The oxidation number of Fe is 3 4 + S O The oxidation number of Sis 2.5 2 4 6 + − S O The oxidation number of Sis 2 2 2 3 + −