
Chapter 7 Redox Reactions and the Base of Electrochemistry X 7.1 The fundamental concepts of redox reactions x 7.2 Electrochemical cells x 7.3 Electrode potentials x 7.4 Application of electrode potentials
Chapter 7 Redox Reactions and the Base of Electrochemistry §7.1 The fundamental concepts of redox reactions §7.2 Electrochemical cells §7.3 Electrode potentials §7.4 Application of electrode potentials

7.1 The fundamental concepts of redox reactions 7.1.1 Oxidation numbers -7.1.2 Balancing redox equations using ion-electron half-reaction method
§ 7.1 The fundamental concepts of redox reactions 7.1.1 Oxidation numbers 7.1.2 Balancing redox equations using ion-electron half-reaction method
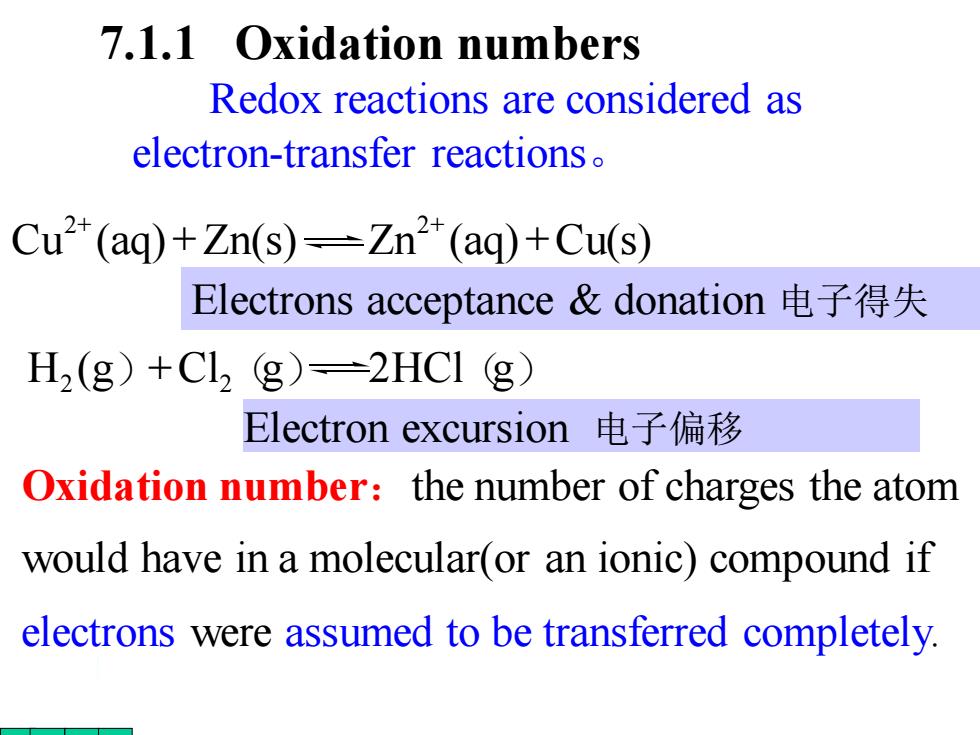
7.1.1 Oxidation numbers Redox reactions are considered as electron-transfer reactions Cu2(aq)+Zn(s)=Zn(aq)+Cu(s) Electrons acceptance&donation电子得失 H2(g)+Cl2 g)=2HCI (g) Electron excursion电子偏移 Oxidation number:the number of charges the atom would have in a molecular(or an ionic)compound if electrons were assumed to be transferred completely
7.1.1 Oxidation numbers Oxidation number:the number of charges the atom would have in a molecular(or an ionic) compound if electrons were assumed to be transferred completely. Redox reactions are considered as electron-transfer reactions。 Cu (aq) Zn(s) Zn (aq) Cu(s) 2 2 + + + + Electron excursion 电子偏移 H2 (g)+Cl2(g) 2HCl(g) Electrons acceptance & donation 电子得失
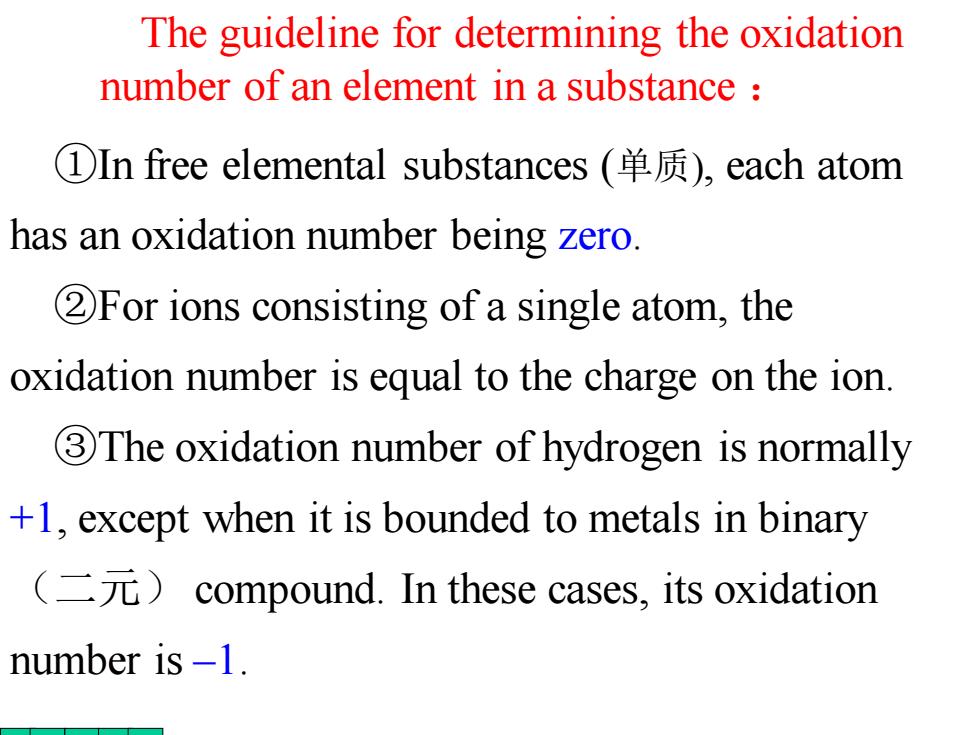
The guideline for determining the oxidation number of an element in a substance ①In free elemental substances(单质),each atom has an oxidation number being zero. 2For ions consisting of a single atom,the oxidation number is equal to the charge on the ion 3 The oxidation number of hydrogen is normally +1,except when it is bounded to metals in binary (二元)compound.In these cases,.its oxidation number is -1
The guideline for determining the oxidation number of an element in a substance : ①In free elemental substances (单质), each atom has an oxidation number being zero. ②For ions consisting of a single atom, the oxidation number is equal to the charge on the ion. ③The oxidation number of hydrogen is normally +1, except when it is bounded to metals in binary (二元) compound. In these cases, its oxidation number is –1

4The oxidation number of oxygen in most compound is-2,but in peroxides(过氧化物) oxygen can have an oxidation number of-1. Oxygen can also have positive oxidation numbers when combined with fluorine.For example,the oxidation number of oxygen is +2 in OF2 and +1 in O2F2
④The oxidation number of oxygen in most compound is –2; but in peroxides(过氧化物) oxygen can have an oxidation number of –1. Oxygen can also have positive oxidation numbers when combined with fluorine. For example, the oxidation number of oxygen is +2 in OF2 and +1 in O2F2
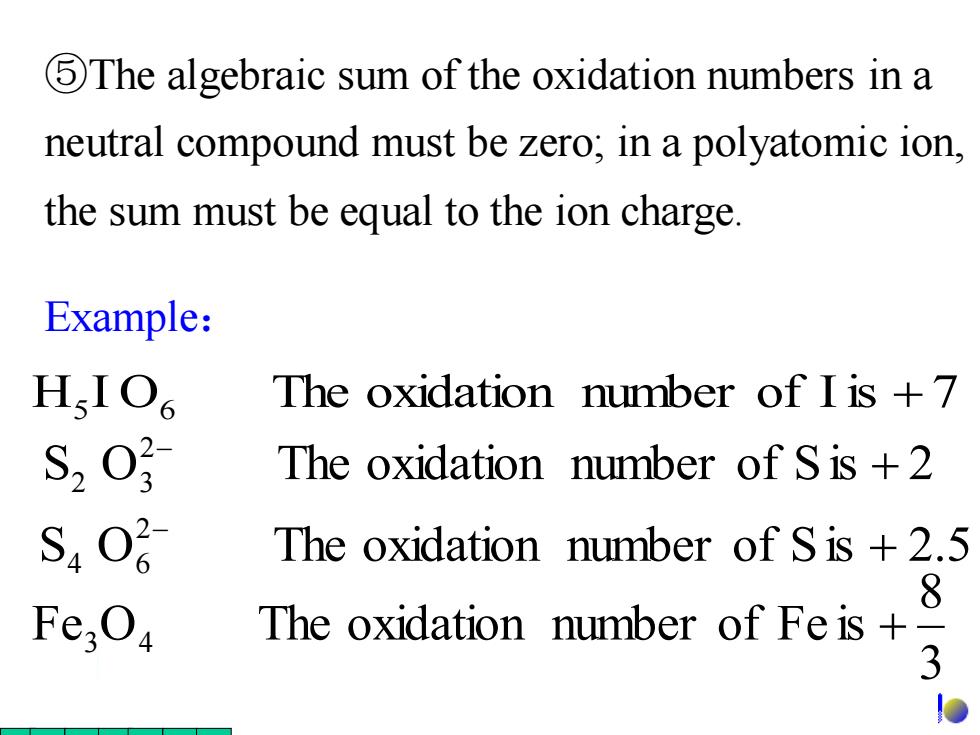
5The algebraic sum of the oxidation numbers in a neutral compound must be zero;in a polyatomic ion, the sum must be equal to the ion charge Example: HIO The oxidation number of I is +7 The oxidation number of S is +2 S40 The oxidation number of S is +2.5 8 Fe;O The oxidation number of Fe is+ 3 D
Example: ⑤The algebraic sum of the oxidation numbers in a neutral compound must be zero; in a polyatomic ion, the sum must be equal to the ion charge. H5 I O6 The oxidation number of Iis + 7 3 8 Fe O The oxidation number of Fe is 3 4 + S O The oxidation number of Sis 2.5 2 4 6 + − S O The oxidation number of Sis 2 2 2 3 + −
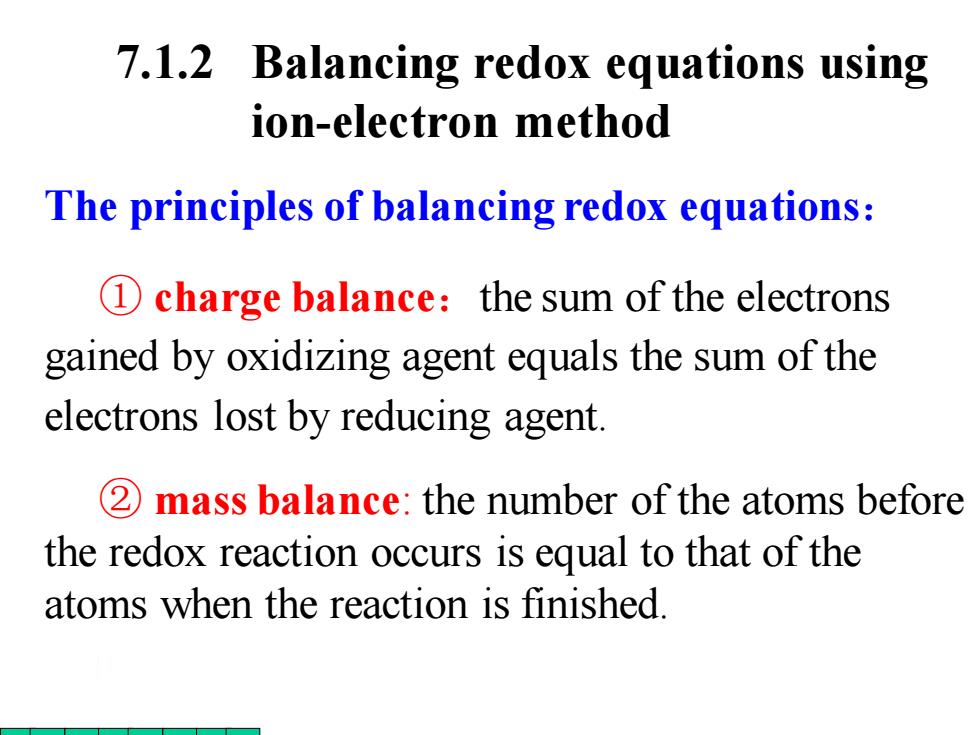
7.1.2 Balancing redox equations using ion-electron method The principles of balancing redox equations: 1 charge balance:the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. 2 mass balance:the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished
The principles of balancing redox equations: ① charge balance:the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. ② mass balance: the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished. 7.1.2 Balancing redox equations using ion-electron method

The general approach to balance a redox equation: 1 write out the unbalanced reaction equation in ionic form (note:gas,pure liquid,solid and weak electrolyte must be written out in molecular form):用离子式写出主要反应 物和产物(气体、纯液体、固体和弱电解质则写分子式)。 2 separate the equation into two half-reactions:the oxidation one and reduction one分别写出氧化剂被还原和还原剂 被氧化的半反应。 3 balance each half-reaction for number and type of atoms and charges分别配平两个半反应方程式,等号两边的各种元素 的原子总数各自相等且电荷数相等
The general approach to balance a redox equation: ① write out the unbalanced reaction equation in ionic form (note: gas, pure liquid, solid and weak electrolyte must be written out in molecular form): 用离子式写出主要反应 物和产物(气体、纯液体、固体和弱电解质则写分子式)。 ② separate the equation into two half-reactions: the oxidation one and reduction one 分别写出氧化剂被还原和还原剂 被氧化的半反应。 ③ balance each half-reaction for number and type of atoms and charges 分别配平两个半反应方程式,等号两边的各种元素 的原子总数各自相等且电荷数相等

4If the oxidation and reduction half-reactions contain different numbers of electrons,we need to multiply one or both half-reactions to equal the number of electrons,then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 Example1-1:Balance the following reaction equation KMnO,(aq)+K2SO3 (aq)MnSO,(aq)+K2SO,(aq)
Example1-1:Balance the following reaction equation ④If the oxidation and reduction half-reactions contain different numbers of electrons, we need to multiply one or both half-reactions to equal the number of electrons, then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 KMnO4 (aq) + K2SO3 (aq) → MnSO4 (aq) + K2SO4 (aq) H+
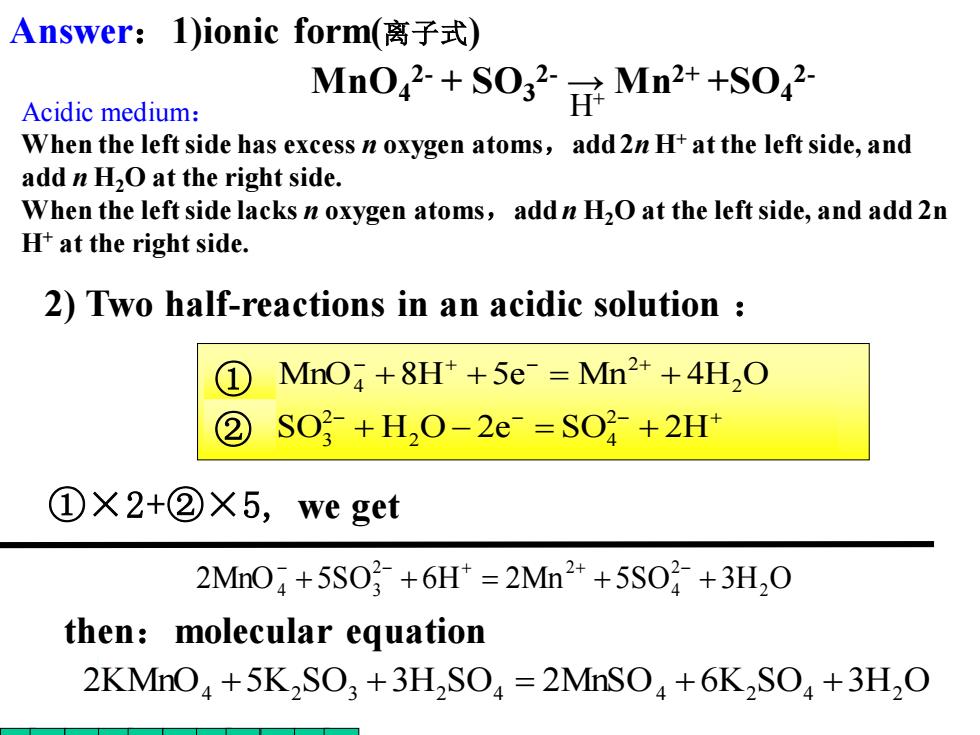
Answer:1)ionic form(离子式) Acidic medium: MnO+OMn2+S0 When the left side has excess n oxygen atoms,add 2n H+at the left side,and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side,and add 2n H+at the right side. 2)Two half-reactions in an acidic solution ① MnO+8H*+5e Mn2++4H,O ② SO2-+H,O-2e-=SO2+2H+ ①X2+②X5,we get 2M04+5S0号+6H=2Mn2++5S0}+3H,0 then:molecular equation 2KMnO+5K2SO3+3H2SO=2MnSO+6K2SO+3H2O
− − − + − + − + + − = + + + = + SO H O 2e SO 2H MnO 8H 5e Mn 4H O 2 2 4 2 3 2 2 ① 4 ② ①×2+②×5, we get 2MnO 5SO 6H 2Mn 5SO 3H2 O 2 4 2 2 4 + 3 + = + + − − + + − 2) Two half-reactions in an acidic solution : 2KMnO4 +5K2 SO3 +3H2 SO4 = 2MnSO4 + 6K2 SO4 +3H2 O then:molecular equation MnO4 2- + SO3 2- → Mn2+ +SO4 2- H+ Answer:1)ionic form(离子式) Acidic medium: When the left side has excess n oxygen atoms,add 2n H+ at the left side, and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side, and add 2n H+ at the right side