
Chapter 10 Reduction oxidization Reactions X 10.1 The fundamental concepts of redox reactions X 10.2 Electrochemical cells x 10.3 Electrode potentials X 10.4 Application of electrode potentials
Chapter 10 Reduction - oxidization Reactions § 10.1 The fundamental concepts of redox reactions § 10.2 Electrochemical cells § 10.3 Electrode potentials § 10.4 Application of electrode potentials

10.1 The fundamental concepts of redox reactions 10.1.1 Oxidation numbers -10.1.2 Balancing redox equations using ion-electron half-reaction method
§ 10.1 The fundamental concepts of redox reactions 10.1.1 Oxidation numbers 10.1.2 Balancing redox equations using ion-electron half-reaction method
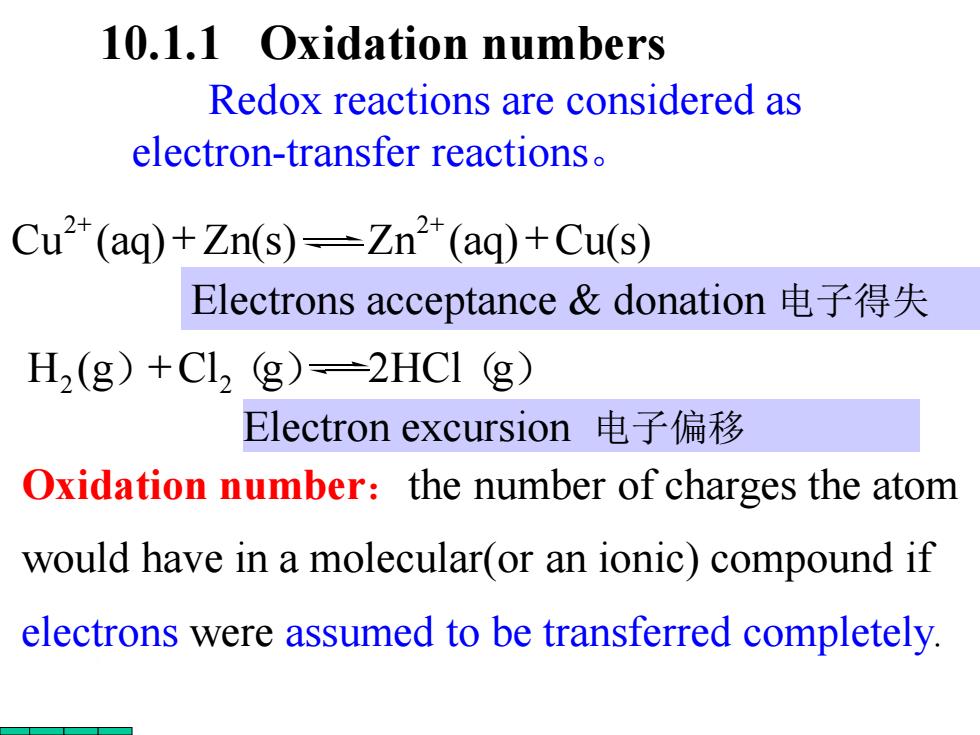
10.1.1 Oxidation numbers Redox reactions are considered as electron-transfer reactions. Cu2(aq)+Zn(s)-Zn2*(aq)+Cu(s) Electrons acceptance&donation电子得失 H2(g)+C12g)=2HC1g) Electron excursion电子偏移 Oxidation number:the number of charges the atom would have in a molecular(or an ionic)compound if electrons were assumed to be transferred completely
10.1.1 Oxidation numbers Oxidation number:the number of charges the atom would have in a molecular(or an ionic) compound if electrons were assumed to be transferred completely. Redox reactions are considered as electron-transfer reactions。 Cu (aq) Zn(s) Zn (aq) Cu(s ) 2 2 + + + + Electron excursion 电子偏移 H2 (g)+Cl2(g) 2HCl(g) Electrons acceptance & donation 电子得失
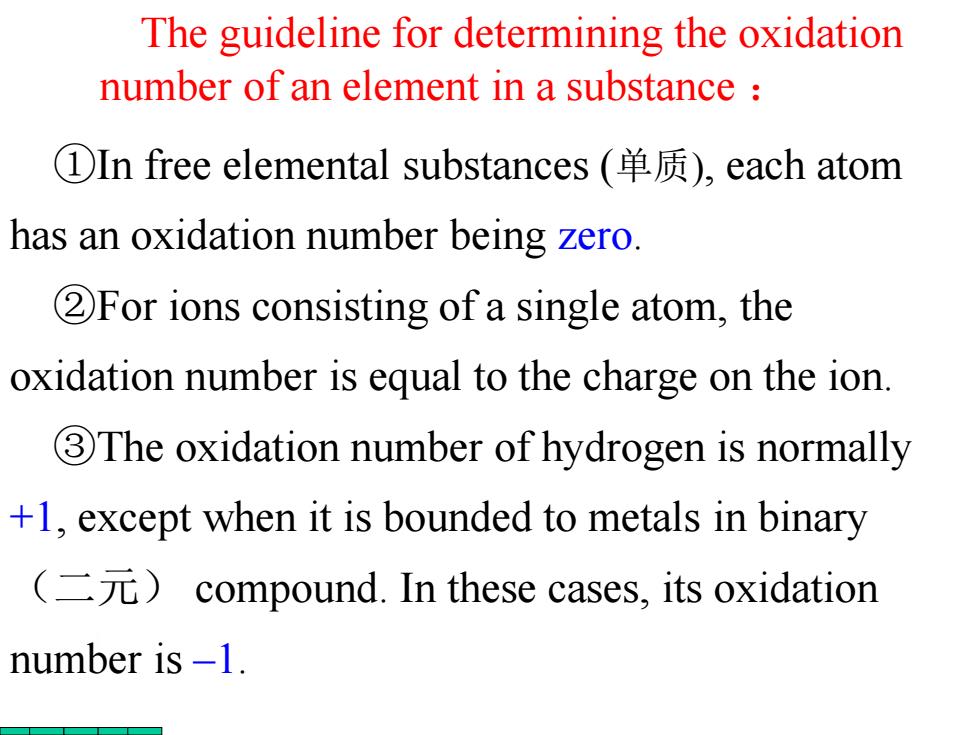
The guideline for determining the oxidation number of an element in a substance ①In free elemental substances(单质),each atom has an oxidation number being zero. 2For ions consisting of a single atom,the oxidation number is equal to the charge on the ion. 3The oxidation number of hydrogen is normally +1,except when it is bounded to metals in binary (二元)compound.In these cases,.its oxidation number is -1
The guideline for determining the oxidation number of an element in a substance : ①In free elemental substances (单质), each atom has an oxidation number being zero. ②For ions consisting of a single atom, the oxidation number is equal to the charge on the ion. ③The oxidation number of hydrogen is normally +1, except when it is bounded to metals in binary (二元) compound. In these cases, its oxidation number is –1

4The oxidation number of oxygen in most compound is-2,but in peroxides(过氧化物) oxygen can have an oxidation number of-1 Oxygen can also have positive oxidation numbers when combined with fluorine.For example,the oxidation number of oxygen is +2 in OF2 and +1 in O2F2
④The oxidation number of oxygen in most compound is –2; but in peroxides(过氧化物) oxygen can have an oxidation number of –1. Oxygen can also have positive oxidation numbers when combined with fluorine. For example, the oxidation number of oxygen is +2 in OF2 and +1 in O2F2
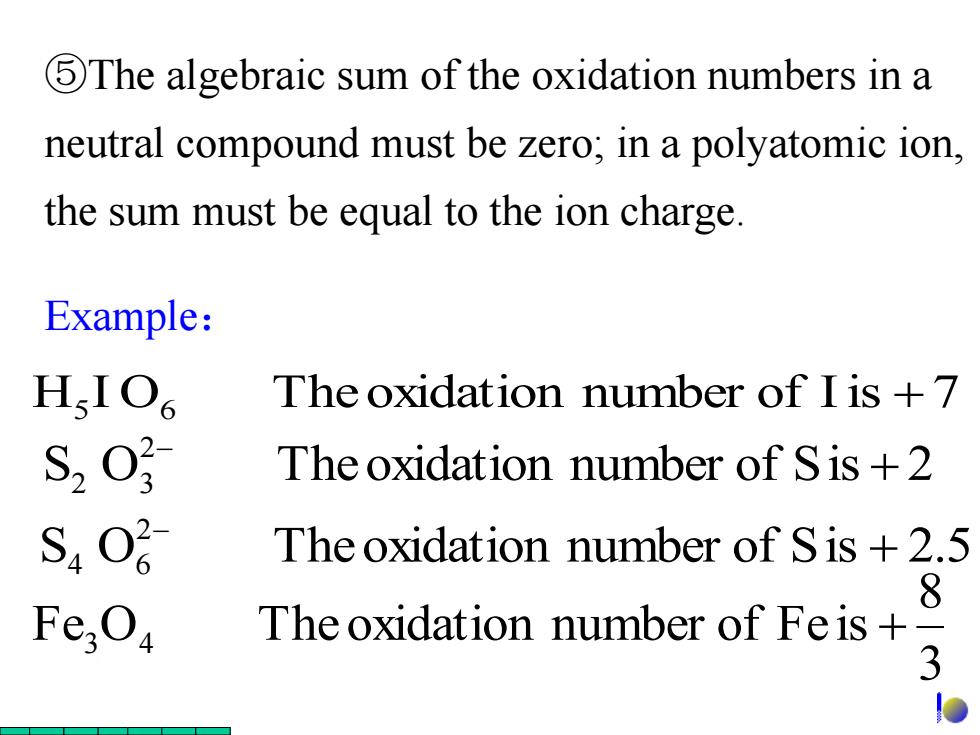
5The algebraic sum of the oxidation numbers in a neutral compound must be zero;in a polyatomic ion the sum must be equal to the ion charge. Example: H.IO The oxidation number of I is +7 S, The oxidation number of Sis +2 S4O2 The oxidation number of Sis +2.5 8 Fe; The oxidation number of Feis+ 3
Example: ⑤The algebraic sum of the oxidation numbers in a neutral compound must be zero; in a polyatomic ion, the sum must be equal to the ion charge. H5 I O6 Theoxidation number of Iis +7 3 8 Fe O Theoxidation number of Fe is 3 4 + S O Theoxidation number of Sis 2.5 2 4 6 + S O Theoxidation number of Sis 2 2 2 3 +
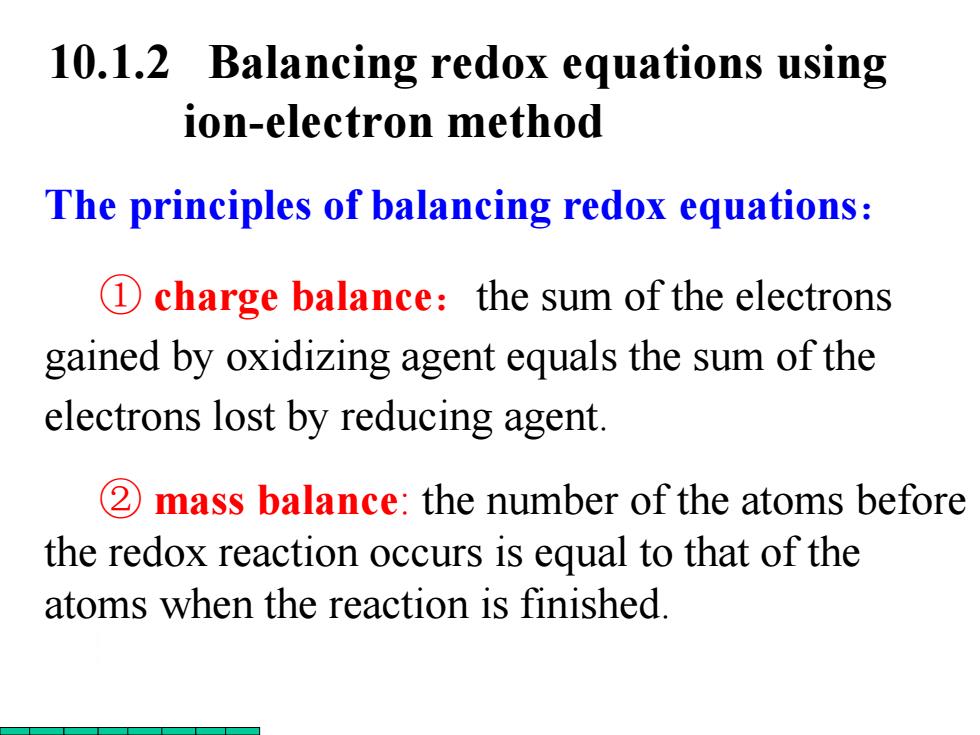
10.1. 2 Balancing redox equations using ion-electron method The principles of balancing redox equations: 1 charge balance:the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. 2 mass balance:the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished
The principles of balancing redox equations: ① charge balance:the sum of the electrons gained by oxidizing agent equals the sum of the electrons lost by reducing agent. ② mass balance: the number of the atoms before the redox reaction occurs is equal to that of the atoms when the reaction is finished. 10.1.2 Balancing redox equations using ion-electron method

The general approach to balance a redox equation: 1write out the unbalanced reaction equation in ionic form(note:gas,pure liquid,solid and weak electrolyte must be written out in molecular form):用离子式写出主要反应 物和产物(气体、纯液体、固体和弱电解质则写分子式)。 2 separate the equation into two half-reactions:the oxidation one and reduction one分别写出氧化剂被还原和还原剂 被氧化的半反应。 3 balance each half-reaction for number and type of atoms and charges分别配平两个半反应方程式,等号两边的各种元素 的原子总数各自相等且电荷数相等
The general approach to balance a redox equation: ① write out the unbalanced reaction equation in ionic form (note: gas, pure liquid, solid and weak electrolyte must be written out in molecular form): 用离子式写出主要反应 物和产物(气体、纯液体、固体和弱电解质则写分子式)。 ② separate the equation into two half-reactions: the oxidation one and reduction one 分别写出氧化剂被还原和还原剂 被氧化的半反应。 ③ balance each half-reaction for number and type of atoms and charges 分别配平两个半反应方程式,等号两边的各种元素 的原子总数各自相等且电荷数相等
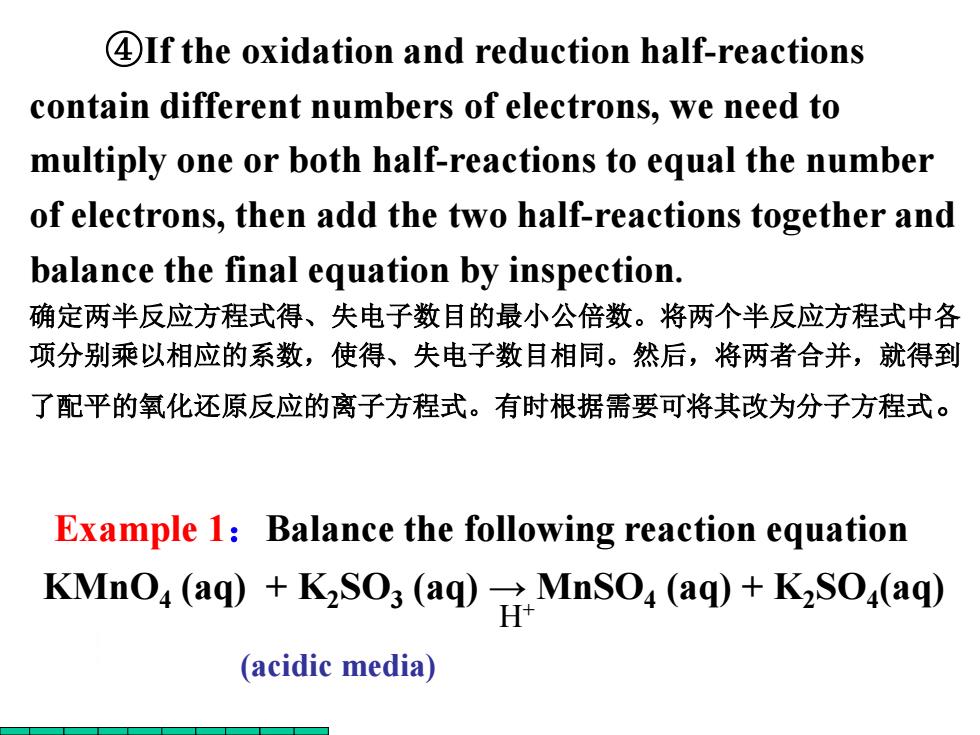
4If the oxidation and reduction half-reactions contain different numbers of electrons,we need to multiply one or both half-reactions to equal the number of electrons,then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 Example 1:Balance the following reaction equation KMnO,(aq)+KSO;(aq)MnSO,(aq)+KSO,(aq) (acidic media)
Example 1:Balance the following reaction equation ④If the oxidation and reduction half-reactions contain different numbers of electrons, we need to multiply one or both half-reactions to equal the number of electrons, then add the two half-reactions together and balance the final equation by inspection. 确定两半反应方程式得、失电子数目的最小公倍数。将两个半反应方程式中各 项分别乘以相应的系数,使得、失电子数目相同。然后,将两者合并,就得到 了配平的氧化还原反应的离子方程式。有时根据需要可将其改为分子方程式。 KMnO4 (aq) + K2SO3 (aq) → MnSO4 (aq) + K2SO4 (aq) H+ (acidic media)
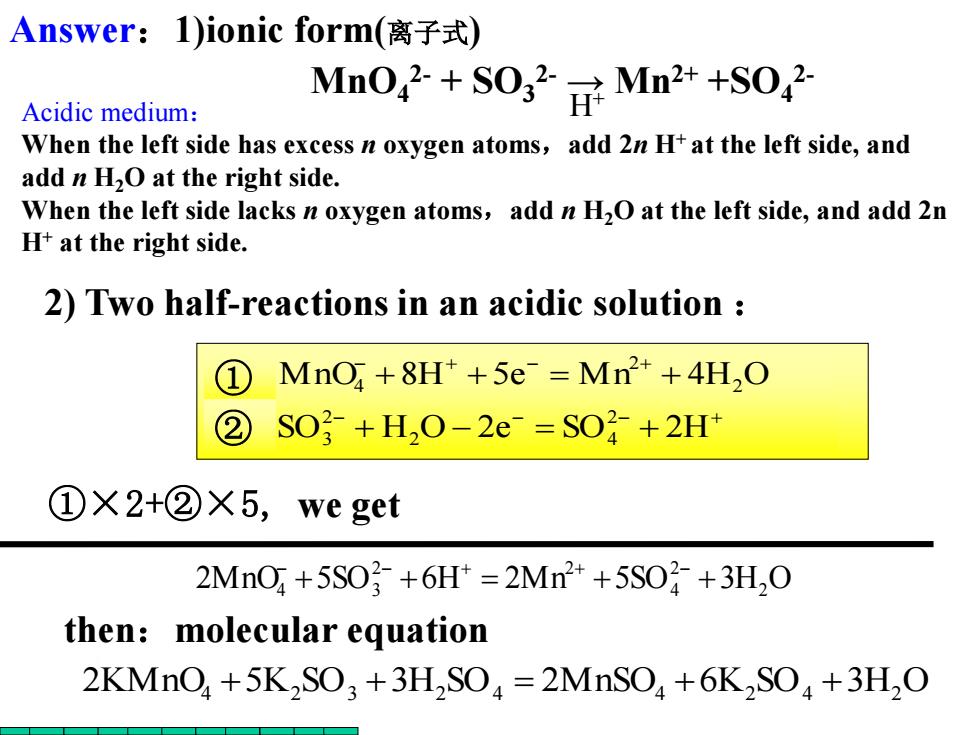
Answer:1)ionic form(离子式) Acidic medium: Mn042+S0g2NMn2++s042 When the left side has excess n oxygen atoms,add 2n H+at the left side,and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side,and add 2n H+at the right side. 2)Two half-reactions in an acidic solution ① MnO,+8H*+5e Mn+4H,O ② SO2+H,O-2e-=SO+2H+ ①×2+②X5,we get 2MnO+5S03+6H=2Mn2++5SO子+3H,0 then:molecular equation 2KMnO+5K2SO3+3H,SO=2MnSO+6K2SO+3H,O
+ + + + + + + + SO H O 2e SO 2H MnO 8H 5e M n 4H O 2 2 4 2 3 2 2 ① 4 ② ①×2+②×5, we get 2MnO 5SO 6H 2Mn 5SO 3H2 O 2 4 2 2 4 + 3 + + + + + 2) Two half-reactions in an acidic solution : 2KMnO4 +5K2 SO3 +3H2 SO4 2MnSO4 +6K2 SO4 +3H2 O then:molecular equation MnO4 2- + SO3 2- → Mn2+ +SO4 2- H+ Answer:1)ionic form(离子式) Acidic medium: When the left side has excess n oxygen atoms,add 2n H+ at the left side, and add n H2O at the right side. When the left side lacks n oxygen atoms,add n H2O at the left side, and add 2n H+ at the right side