
Chapter 11 Basic of Coordination Chemistry 11.1 Basic concepts of coordination compounds 11.2 The valence bond theory of coordination compounds 11.3 Crystal Field Theoryof coordination compound 11.4 Stability of coordination compounds $*11.5 Further discussion on coordination compounds
Chapter 11 Basic of Coordination Chemistry §11.2 The valence bond theory of coordination compounds §11.3 Crystal Field Theoryof coordination compound §11.1 Basic concepts of coordination compounds §*11.5 Further discussion on coordination compounds §11.4 Stability of coordination compounds
S 11.1 Basic concepts of coordination compounds 11.1.1 Coordination compounds Defination: A complex is a typical acid-base adduct of a Lewis acid and a lewis base.E.g,[AgNH3)2]+是Lewisi酸Ag+和NH3 的加合物。 A coordination compound or metal complex,has a structure consisting of a central atom or ion (usually metallic),bonded to a surrounding array of molecules or anions (ligands,complexing agents)
11.1.1 Coordination compounds Defination: A complex is a typical acid-base adduct of a Lewis acid and a lewis base. E.g., [Ag(NH3)2]+是Lewis酸Ag+和NH3 的加合物。 §11.1 Basic concepts of coordination compounds A coordination compound or metal complex, has a structure consisting of a central atom or ion (usually metallic), bonded to a surrounding array of molecules or anions (ligands, complexing agents)
Composition of a coordination A Lewis acid,.called形成体(eg,central metal ion): A Lewis base,.called配位体(ligand). Central metal ion(形成体)usually are metals and metal ions,also have few nonmelal .Such as Cu2+,Agt,Fe3+, Fe,Ni,Bm,PV.… Ligand(配位体)in classical coordination chemistry bind to metals,almost exclusively,via their "lone pairs"of electrons residing on the main group atoms of the ligand. Typical ligands are:F-,CI,Br,I,OH-,CN-,H2O, NH3,:C0,H2C=CH2.…
Central metal ion(形成体) usually are metals and metal ions,also have few nonmelal .Such as :Cu2+,Ag+ ,Fe3+, Fe,Ni,BⅢ,PⅤ …… Ligand(配位体) in classical coordination chemistry bind to metals, almost exclusively, via their “lone pairs” of electrons residing on the main group atoms of the ligand. Typical ligands are:F-, Cl-, Br-, I-, OH-, CN- , H2O, NH3 , :CO, H2C=CH2…… A Lewis acid, called形成体(e.g., central metal ion); A Lewis base, called 配位体 (ligand). Composition of a coordination

coordinating atom:The atom having bond with the central metal ion. monodentate ligand:There is only one coordination atom in the ligand. Polydentate ligand:The ligand have two or more coordination atoms
coordinating atom:The atom having bond with the central metal ion. monodentate ligand:There is only one coordination atom in the ligand. Polydentate ligand:The ligand have two or more coordination atoms

Example 乙二酸根(草酸根)C,O 乙二胺四乙酸根EDTA(Y4-) OOC-HC CH-co] N-CH,-CH,-N: OOC-H,C CH,-COO
乙二胺四乙酸根 EDTA(Y4-) 乙二酸根(草酸根) 2 C2O4 O O C C O O 2– • • • • COO H C CH OOC N CH CH N COO H C CH OOC 2 2 2 2 2 2 4– • • • • • • • • • • • • Example

从溶液中析出配合物时,配离子常与带有相反电 荷的其它离子结合成盐,这类盐称为配盐(complex salt)。配盐的组成可以划分为内层(inner sphere)和外 层(outer sphere)。配离子属于内层,配离子以外的其 他离子属于外层。外层离子所带电荷总数等于配离子 的电荷数。 out sphere inner sphere K3[Fe(CN)6] ↑ 形成体 配配配 位体位 原子
从溶液中析出配合物时,配离子常与带有相反电 荷的其它离子结合成盐,这类盐称为配盐(complex salt)。配盐的组成可以划分为内层(inner sphere)和外 层(outer sphere)。配离子属于内层,配离子以外的其 他离子属于外层。外层离子所带电荷总数等于配离子 的电荷数。 形 成 体 配 位 原 子 配 体 配 位 数 K3[ Fe ( C N ) 6 ] out sphere inner sphere
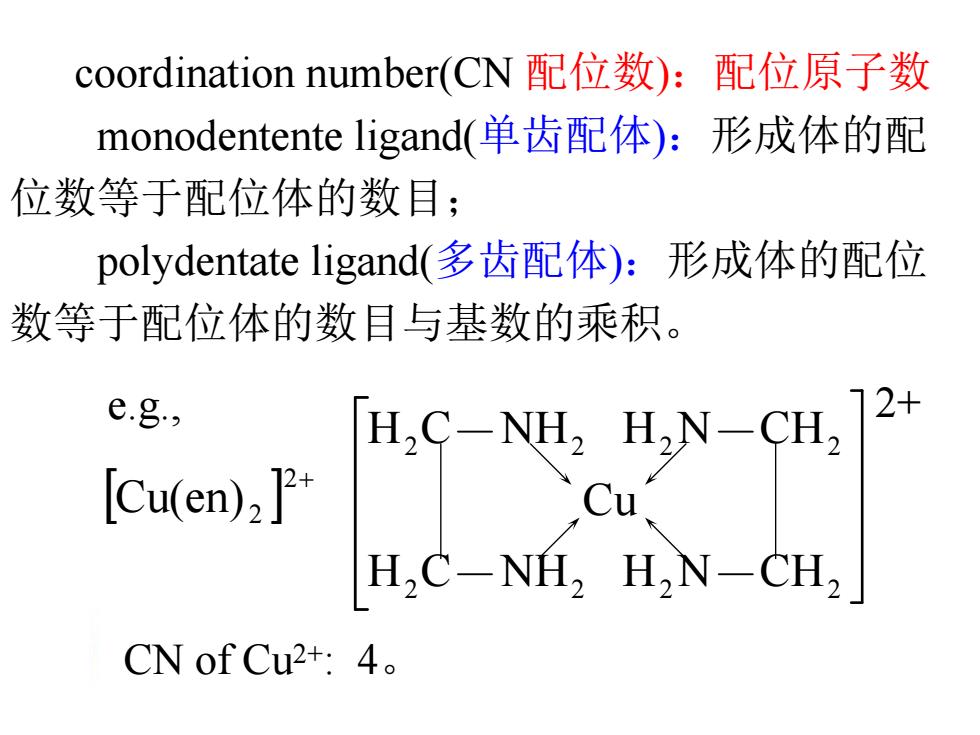
coordination number(CN配位数):配位原子数 monodentente ligand(单齿配体):形成体的配 位数等于配位体的数目; polydentate ligand(多齿配体):形成体的配位 数等于配位体的数目与基数的乘积。 e.g., [Cu(en)2] H.C-NEL.HN CU. Cu H,C-NH,H,N-CH, CN of Cu2+:4
coordination number(CN 配位数):配位原子数 monodentente ligand(单齿配体):形成体的配 位数等于配位体的数目; polydentate ligand(多齿配体):形成体的配位 数等于配位体的数目与基数的乘积。 Cu(en) 2 2 CN of Cu2+: 4。 e.g., H C NH H N CH Cu H C NH H N CH 2 2 2 2 2 2 2 2 2+

[Ca(EDTA)2 or CaY2- CN of Ca2+:6, CH, the coordinating atoms are 4 O,2 N atoms respectively
[Ca(EDTA)]2- or CaY2- CN of Ca2+: 6, the coordinating atoms are 4 O,2 N atoms respectively

11.1.2.Names of complexes of various types l.The basic procedure for naming a complex(英语命名 法): 1When naming a complex ion,the ligands are named before the metal ion. 2Write the names of the ligands in alphabetical order. i)multiple occurring monodentate ligands receive a prefix according to the number of occurrences:di-,tri-, tetra-,penta-,or hexa.Polydentate ligands (e.g., ethylenediamine,oxalate)receive bis-,tris-,tetrakis-,etc. ii)anions end in o.This replaces the final 'e'when the anion ends with '-ate',e.g.sulfate becomes sulfato.It replaces 'ide':cyanide becomes cyano
11.1.2. Names of complexes of various types 1. The basic procedure for naming a complex (英语命名 法): ①When naming a complex ion, the ligands are named before the metal ion. ②Write the names of the ligands in alphabetical order. ⅰ) multiple occurring monodentate ligands receive a prefix according to the number of occurrences: di-, tri-, tetra-, penta-, or hexa. Polydentate ligands (e.g., ethylenediamine, oxalate) receive bis-, tris-, tetrakis-, etc. ⅱ) anions end in o. This replaces the final 'e' when the anion ends with '-ate', e.g. sulfate becomes sulfato. It replaces 'ide': cyanide becomes cyano

iii)neutral ligands are given their usual name,with some exceptions:NH3 becomes ammine;H2O becomes aqua or aquo;CO becomes carbonyl;NO becomes nitrosyl. 3 write the name of the central atom/ion.If the complex is an anion,the central atom's name will end in -ate,and its Latin name will be used if available (except for mercury). 4If the central atom's oxidation state needs to be specified (when it is one of several possible,or zero),write it as a Roman numeral (or 0)in parentheses. 5Name cation then anion as separate words(if applicable,as in last example)
ⅲ) neutral ligands are given their usual name, with some exceptions: NH3 becomes ammine; H2O becomes aqua or aquo; CO becomes carbonyl; NO becomes nitrosyl. ③ write the name of the central atom/ion. If the complex is an anion, the central atom's name will end in -ate, and its Latin name will be used if available (except for mercury). ④If the central atom's oxidation state needs to be specified (when it is one of several possible, or zero), write it as a Roman numeral (or 0) in parentheses. ⑤Name cation then anion as separate words (if applicable, as in last example)