
Chapter 4 Chemical equilibria, entropy and Gibbs function §4.1 The standard equilibrium constant §4.2 The application of the standard equilibrium constant 4.3 The shift of a chemical equilibrium 4.4 Spontaneous reactions and entropy §4.5 Gibbs function

4.1 The standard equilibrium constant 4.1.1 The basic features of a chemical equilibrium 4.1.2 The standard equilibrium constant expression 4.1.3 Experimental measurement of the standard equilibrium constant
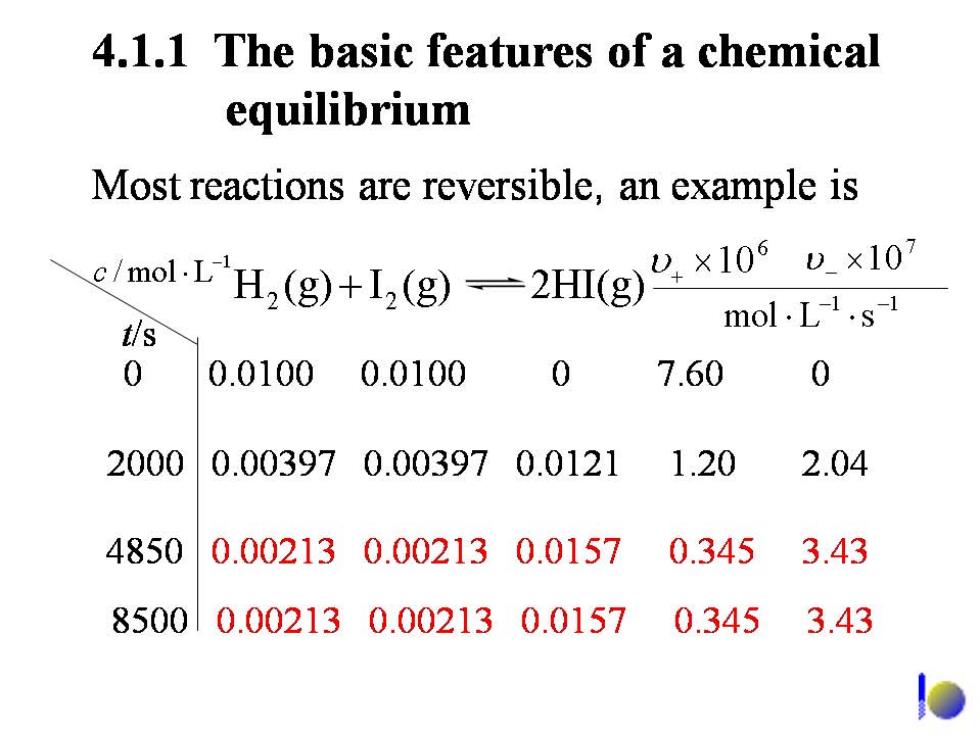
4.1.1 The basic features of a chemical equilibrium Most reactions are reversible,an example is en-LH,(g+1,(g一2H(g”×10°u×10 t/s mol.L1.s-1 0 0.01000.0100 0 7.60 0 2000 0.003970.003970.0121 1.20 2.04 4850 0.002130.002130.0157 0.345 3.43 85000.002130.002130.01570.3453.43
Initially,concentrations of H,and I,are large,only the forward reaction occurs;As time passes,the concentrations of H,and I2 decrease and in result the forward reaction slows down.The concentrations of HI increase and in result the reverse reaction speeds up. The reaction mixture is at equilibrium until the forward and reverse reactions go at the same rate. lo

05 (7ow) HI 0逆 H2,lz t/s H,(g)+I,(g)=2HⅡ(g) 550 HI 2HI(g)≠H2(g)+2(g) H2,h t/s

A chemical equilibrium: A reversible chemical reaction can be at equilibrium under certain conditions: D正=D道≠0 The basic features of chemical equilibrium: (1)the composition of an equilibrium mixture undergoes no further change with time. (2)chemical equilibria are dynamic. (3)the equilibrium composition is independent of the path of approach

4.1.2 The standard equilibrium constant expression *Equilibria involving gases H2(g)+I2(g)=2HI(g) [p(HI)/p2 K= pe=100kpa [p(H2)/pe]p(I,)/pe] *Equilibria in aqueous solution: Sn2(aq)+2Fe3+(aq)-Sn4+(aq)+2Fe2+(aq) ke_Lc(Sn "c)lle(Fc2)2 [c(Sn 2/ce )l[c(Fe 3*/c)] ce=1 mol.H1
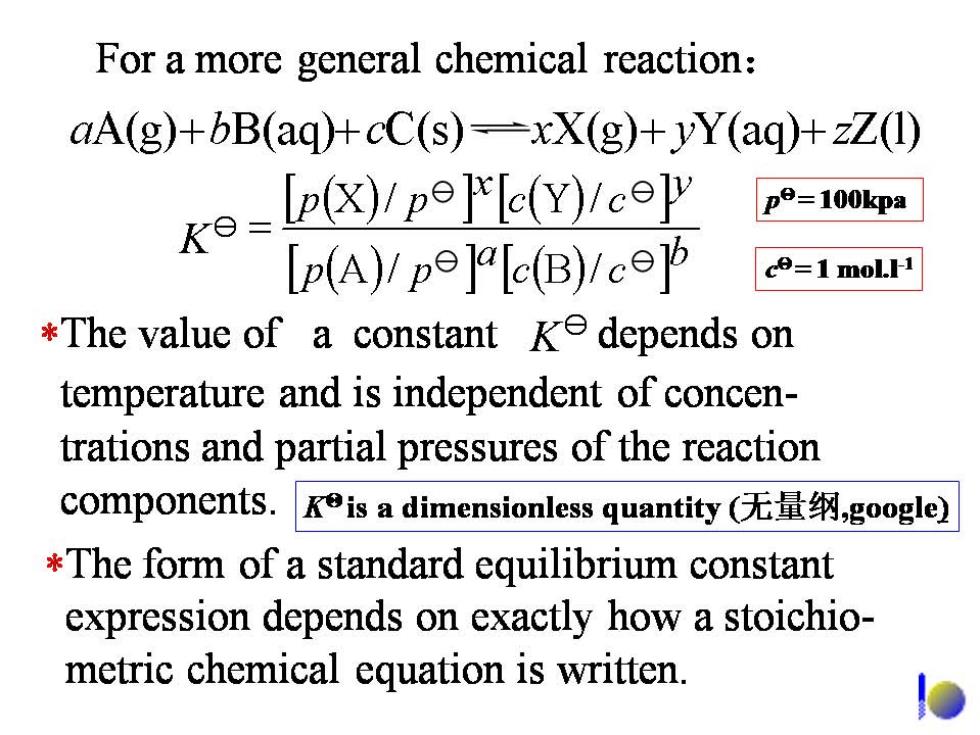
For a more general chemical reaction: aA(g)+bB(aq)+cC(s)=xX(g)+yY(aq)+zZ(1) Ke=pyp9]'eY/ceΨ pe=100kpa [p(A)/pe]r[c(B)1co的 co=1 molH *The value of a constant ke depends on temperature and is independent of concen- trations and partial pressures of the reaction components.Kis a dimensionless quantity(无量纲,google) *The form of a standard equilibrium constant expression depends on exactly how a stoichio- metric chemical equation is written
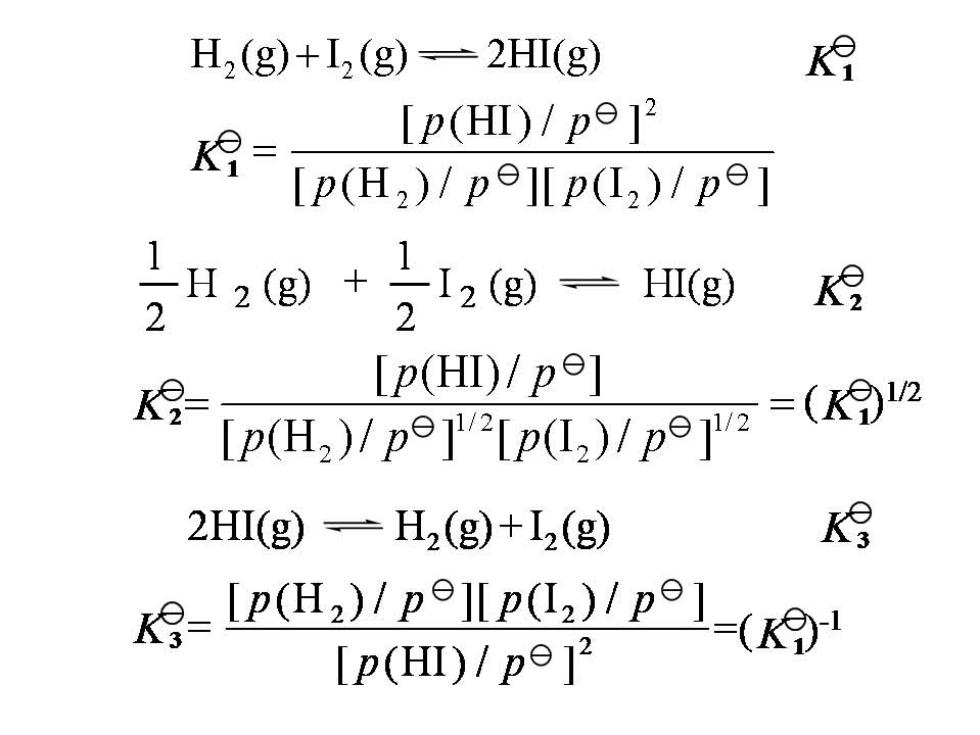
H2(g)+I(g)=2HΠ(g) [p(HⅢ)/po12 [p(H,)/pelp(I2)/pe] H2g)+号12g一Hg) 月 2 2 [p(HI)/p] Ip(H)p( 2HI(g)=H2(g)+L2(g) 月 -PCH)/P1-( [P(HI)/p]
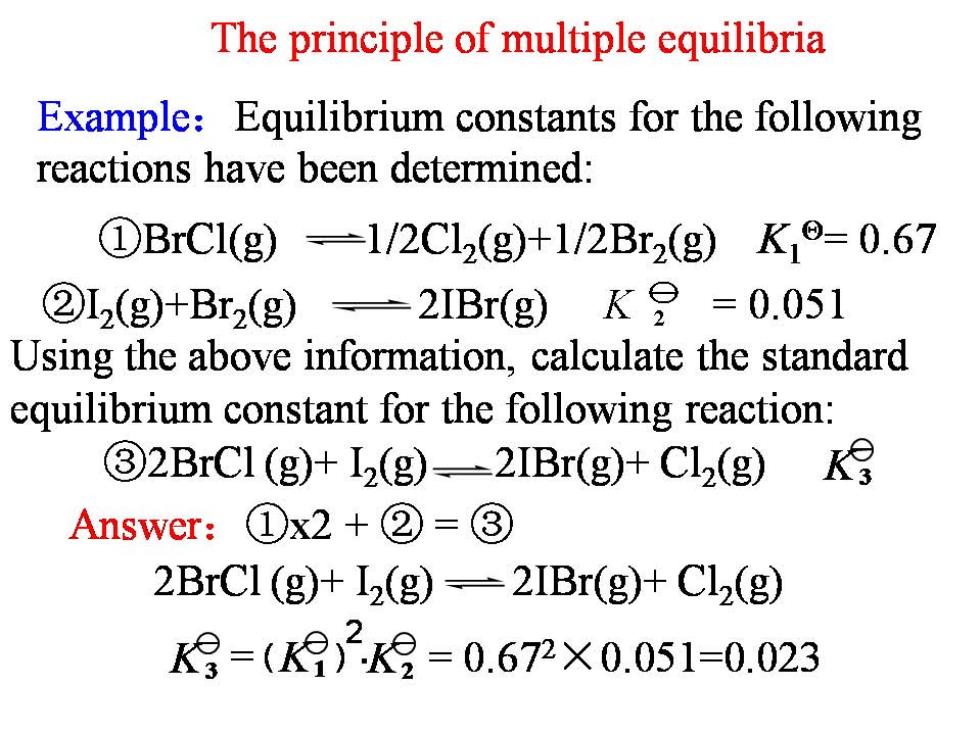
The principle of multiple equilibria Example:Equilibrium constants for the following reactions have been determined: ①BrC1(g)=1/2Cl2(g+1/2Br2(gK19=0.67 ②L2(g)+Br2(g)=2IBr(g)K9=0.051 Using the above information,calculate the standard equilibrium constant for the following reaction: 32BrCl (g)+I2(g)-2IBr(g)+Cl2(g) Answer:①x2+②=③ 2BrCl(g)+I2(g)=2IBr(g)+Cl2(g) K9=()2=0.672×0.051=0.023