
Chapter 12 The s-Block Elements 12.1 The general properties of the s-block elements X 12.2 The elementary substances קl2.3 The properties of the alkali and alkaline earth compounds X$12.4 The particularity of Li and Be The diagonal relationship
§12.1 The general properties of the s-block elements §12.4 The particularity of Li and Be The diagonal relationship §12.3 The properties of the alkali and alkaline earth compounds §12.2 The elementary substances Chapter 12 The s-Block Elements
§12.1 The general properties of the s-block elements The alkali metals(IA )ns Li,Na,K,Rb,Cs,Fr The alkaline metals (IIA )ns2 Be,Mg,Ca,Sr,Ba,Ra They are all active metals e
§12.1 The general properties of the s-block elements The alkali metals (IA ):ns1 Li, Na, K, Rb, Cs, Fr The alkaline metals (IIA ):ns2 Be, Mg, Ca, Sr, Ba, Ra They are all active metals

IA IIA electronegativity Decreasing g ionization energy and Increasing metallicity Increasing atomic Li Be Na Mg K Ca Rb Sr radius Cs Ba reductibility Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity
electronegativity Decreasing ionization energy and Increasing metallicity and reductibility Increasing atomic radius Decreasing atomic radius Decreasing metallicity and reductibility Increasing ionization energy and electronegativity IA IIA Li Be Na Mg K Ca Rb Sr Cs Ba
General properties of S block elemental substancs 1.React with H2 to produce ionic compounds M+1H-1, M+2H-12 (except for Be); 2.React with O,to form oxide,peroxide,superoxide; 3.React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4.Dissolve into liquid NH3 to form blue reducing solution except for Be Note:they show different activities
1. React with H2 to produce ionic compounds M+1H-1、 M+2H-12 (except for Be); 2. React with O2 to form oxide, peroxide, superoxide; 3. React with water and non-metallic elements to form the corresponding compounds except for Be and Mg; 4. Dissolve into liquid NH3 to form blue reducing solution except for Be General properties of S block elemental substancs Note: they show different activities

S 12.2 The elementary substances 12.2.1 The properties of the elementary substances 1.Physical properties ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; *Good conductor of heat and electricity Na K
§12.2.1 The properties of the elementary substances Na Li K 1.Physical properties §12.2 The elementary substances ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; ☆Good conductor of heat and electricity

Rb Cs 181 Low melting point Be Mg a Sr Ba Li Na KRb Cs Fr
Be Mg Ca Sr Ba Rb Cs Low melting point Li Na K Rb Cs Fr

2.Chemical properties: ·React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. The elementary substances form the corresponding oxides when they burn in air: LiO Na202 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 Na,O2 Li,C Pale yellow K Pale yellow Magnesium burning in air
The elementary substances form the corresponding oxides when they burn in air: Li2O Na2O2 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 • React with oxygen、sulfur、nitrogen and halogen to form the corresponding compounds. 2.Chemical properties: Magnesium burning in air Li2O Na2O2 KO2 Pale yellow Pale yellow

.React with water 2M+2H,O-2MOH+H2(g) Li Na K Bromothymol blue indicator 溴百里酚兰指示剂 Ca
•React with water Li Na K Ca 2M + 2H 2O → 2MOH + H 2(g) Bromothymol blue indicator 溴百里酚兰 指示剂
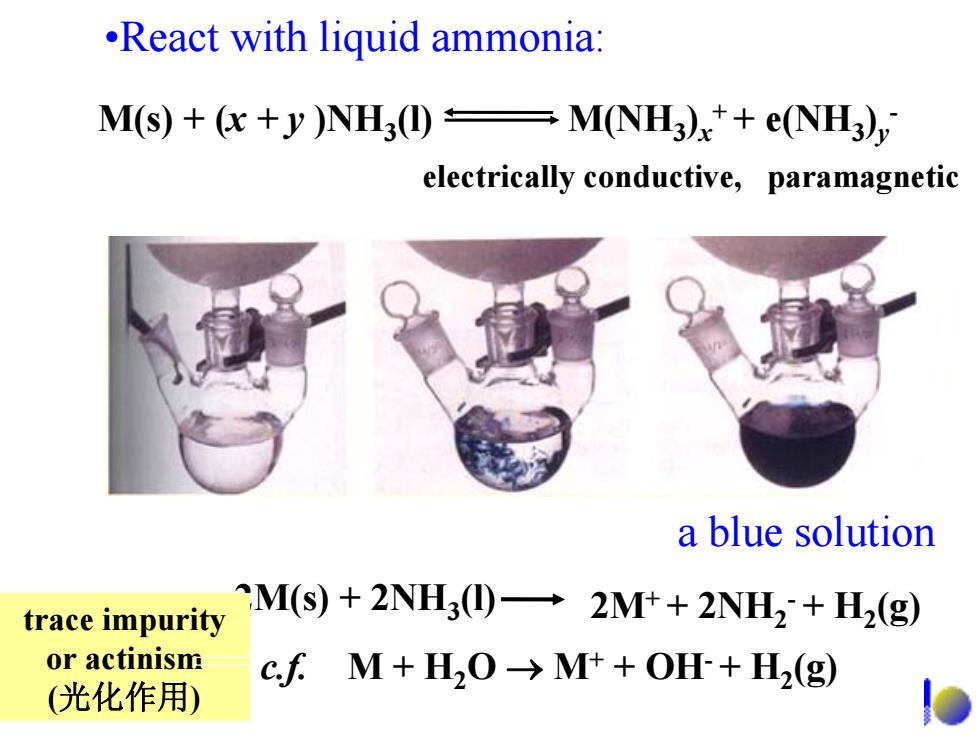
.React with liquid ammonia: M(s)+(x+y)NH3(I)M(NH3)x++e(NH3), electrically conductive,paramagnetic a blue solution trace impurity CM(s)+2NH3(I)-2M++2NH2+H2(g) or actinism cfM+H2O→Mt+OH+H2(g) (光化作用)
•React with liquid ammonia: a blue solution 2M(s) + 2NH 3(l) 2M+ + 2NH 2 - + H 2(g) c.f. M + H 2O → M + + OH - + H 2(g) M(s) + (x + y )NH 3(l) M(NH 3 ) x + + e(NH 3 )y - electrically conductive, paramagnetic trace impurity or actinism (光化作用 )
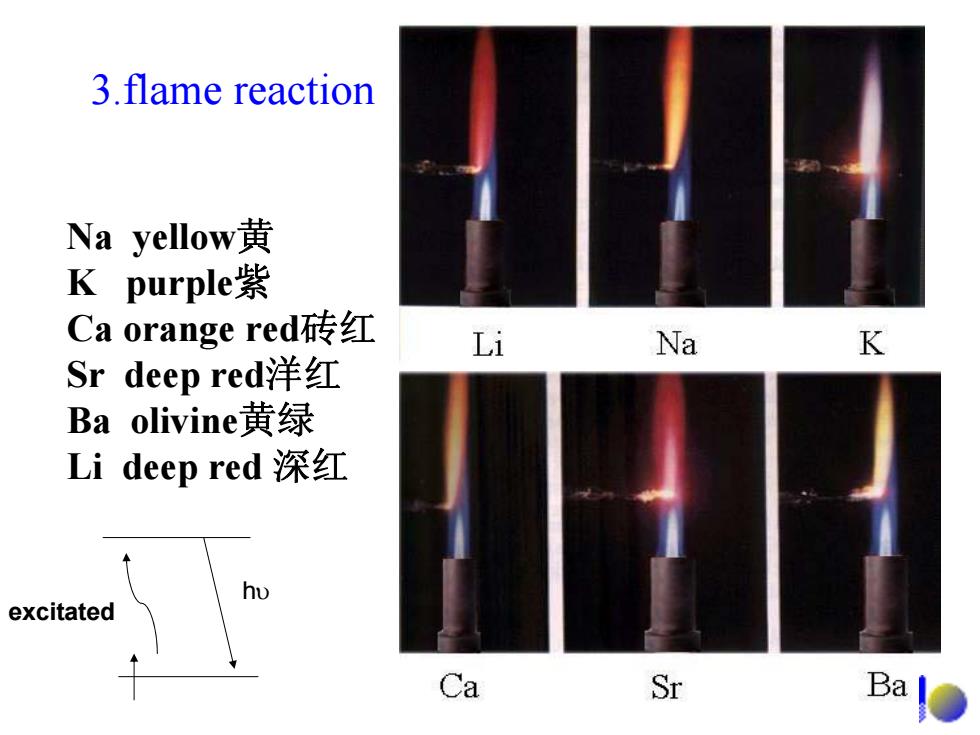
3.flame reaction Na yellow黄 K purple紫 Ca orange red砖红 Li Na K Sr deep red洋红 Ba olivine黄绿 Li deep red深红 excitated Ca Sr Ba
3.flame reaction Na yellow 黄 K purple 紫 Ca orange red砖红 Sr deep red洋红 Ba olivine黄绿 Li deep red 深红 excitated h υ