
Chapter 5 Acid-Base Equilibrium X$5.1 The Bronsted theory of acids and bases ק5.2 Ionization equilibrium of water and the pH scale X$5.3 Equilibrium in solutions of weak acids and weak bases X$5.4 Buffer solutions
Chapter 5 Acid-Base Equilibrium § 5.4 Buffer solutions § 5.3 Equilibrium in solutions of weak acids and weak bases § 5.2 Ionization equilibrium of water and the pH scale § 5.1 The Brønsted theory of acids and bases
X 5.5 Acid-bases indicators X$5.6 Lewis acids and bases and coordination compounds x 5.7 Complexation reaction and coordination equilibrium
§ 5.7 Complexation reaction and coordination equilibrium § 5.6 Lewis acids and bases and coordination compounds § 5.5 Acid-bases indicators

5.1 The Bronsted theory of acids and bases 5.1.1 The Bronsted acids and bases -5.1.2 Relative strengths of acids and bases
§ 5.1 The Brønsted theory of acids and bases 5.1.2 Relative strengths of acids and bases 5.1.1 The Brønsted acids and bases

Overview::(sour,bitter)Taste-→Arrhenius theory(酸碱电离理论, H+&OHr),→Solvent theory,.Bronsted theory(质子理论),Lewis theory(电子理论),soft-hard acid/.base theory.. 5.1.1 The Bronsted theory of acids and bases(酸碱质子理论) acid:a substance capable of donating a proton.(proton donor) (质子的给予体) base:a substance that can accept a proton.(proton acceptor) (质子的接受体)
acid: a substance capable of donating a proton. (proton donor) (质子的给予体) base: a substance that can accept a proton. (proton acceptor) (质子的接受体) 5.1.1 The Brønsted theory of acids and bases (酸碱质子理论) Overview: (sour, bitter) Taste→ Arrhenius theory(酸碱电离理论, H+&OH-), → Solvent theory, Bronsted theory(质子理论), Lewis theory(电子理论), soft-hard acid/base theory…
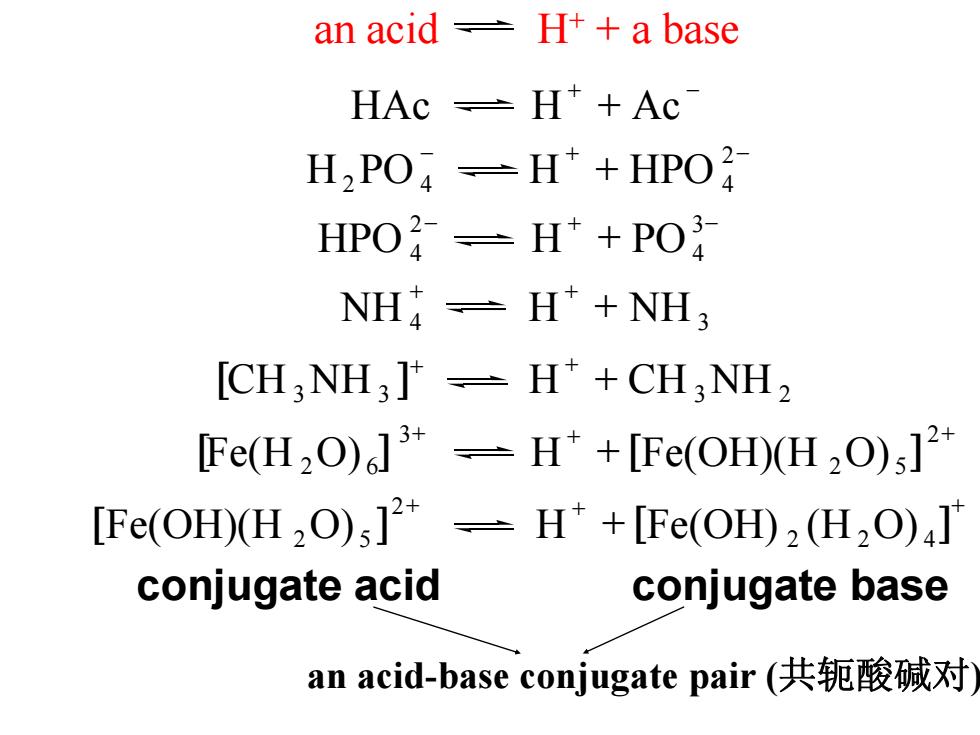
an acid H++a base HAc -H+Ac H2 PO=H*+HPO2 HPO2-H*+PO NH=H +NH3 [CH,NH3]-H*+CH3 NH2 Fe(H2O)]-H*+[Fe(OH)(H2O);]2 [Fe(OH)(H2O)s]*-H*+[Fe(OH)2(H2O)J conjugate acid conjugate base an acid-base conjugate pair(共轭酸碱对
an acid H+ + a base + − HAc H + Ac − + − + 2 H2PO 4 H HPO 4 − + − + 34 2 HPO 4 H PO + + + NH 4 H NH 3 [ ]+ + + CH 3NH 3 H CH 3NH 2 + + + + 2 2 5 3 [] [ ] Fe(H 2O) 6 H Fe(OH)(H O) + + + + 2 2 4 2 [ ] Fe(OH)(H 2O) 5 H [ ] Fe(OH) (H O) conjugate acid conjugate base an acid-base conjugate pair (共轭酸碱对)
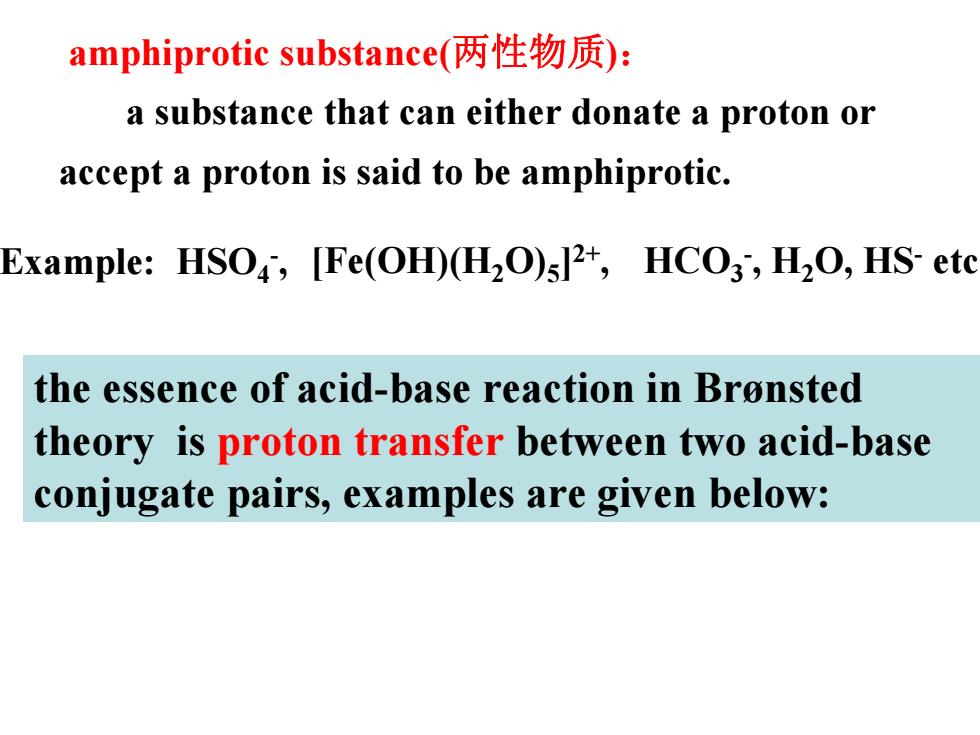
amphiprotic substance(两性物质): a substance that can either donate a proton or accept a proton is said to be amphiprotic. Example:HSO,[Fe(OH)(H2O)512,HCO3,H2O,HS-etc the essence of acid-base reaction in Bronsted theory is proton transfer between two acid-base conjugate pairs,examples are given below:
amphiprotic substance(两性物质 ) : a substance that can either donate a proton or accept a proton is said to be amphiprotic. HCO 3 -, H 2O, HS - [Fe(OH)(H etc 2O) 5 ]2+ HSO , 4 - Example: , the essence of acid-base reaction in Brønsted theory is proton transfer between two acid-base conjugate pairs, examples are given below:
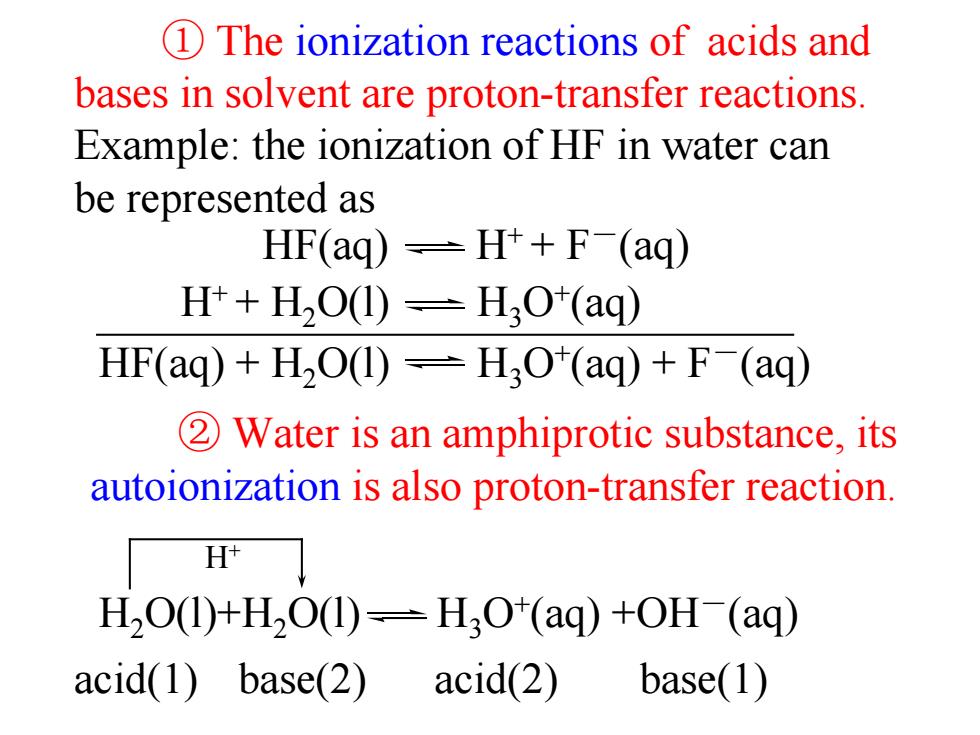
1 The ionization reactions of acids and bases in solvent are proton-transfer reactions. Example:the ionization of HF in water can be represented as HF(ag)-H++F(aq) H++H2O(1)-H;O*(aq) HF(aq)+H2O(1)=H3O*(aq)+F(aq) 2Water is an amphiprotic substance,its autoionization is also proton-transfer reaction. H H2O(1)+H2O(1)=H3O*(aq)+OH-(aq) acid(1)base(2) acid(2) base(1)
① The ionization reactions of acids and bases in solvent are proton-transfer reactions. Example: the ionization of HF in water can be represented as ② Water is an amphiprotic substance, its autoionization is also proton-transfer reaction. H+ acid(1) base(2) acid(2) base(1) HF(aq) H+ + F-(aq) H+ + H2O(l) H3O+(aq) HF(aq) + H2O(l) H3O+(aq) + F-(aq) H2O(l)+H2O(l) H3O+(aq) +OH-(aq)
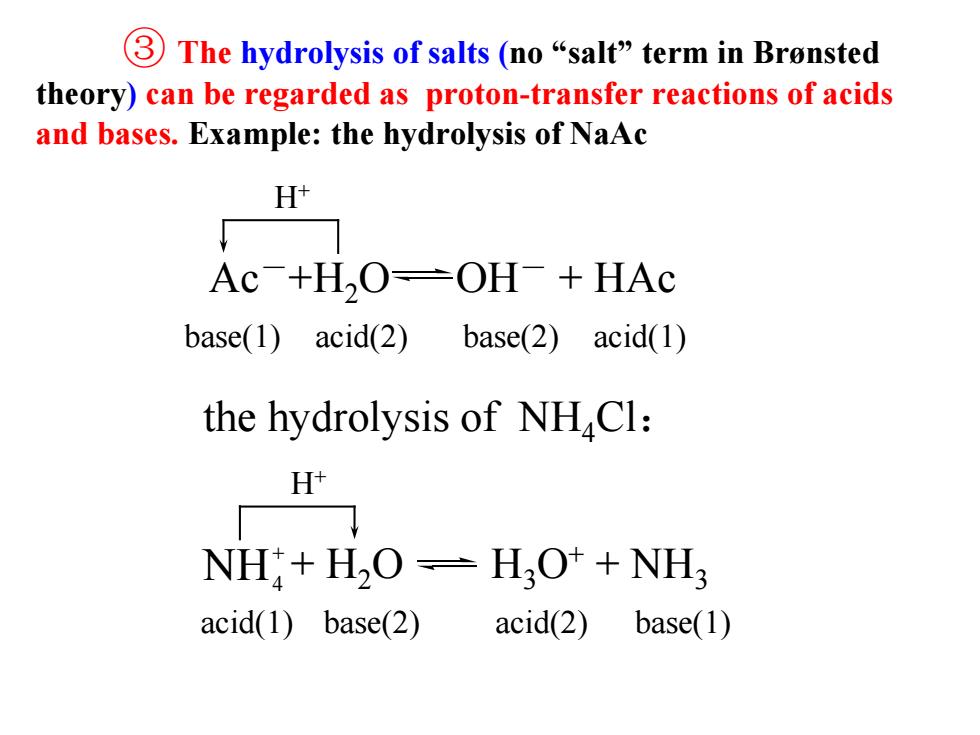
③The hydrolysis of salts(no“salt”term in Bronsted theory)can be regarded as proton-transfer reactions of acids and bases.Example:the hydrolysis of NaAc H Ac+H,O-OH+HAc base(1)acid(2)base(2)acid(1) the hydrolysis of NHCl: H NH+H2O=H2O++NH acid(1)base(2) acid(2) base(1)
③ The hydrolysis of salts (no “salt” term in Brønsted theory) can be regarded as proton-transfer reactions of acids and bases. Example: the hydrolysis of NaAc base(1) acid(2) base(2) acid(1) H+ the hydrolysis of NH4Cl: acid(1) base(2) acid(2) base(1) H+ Ac-+H2O OH- + HAc + H2O H3O+ + NH3 + NH4
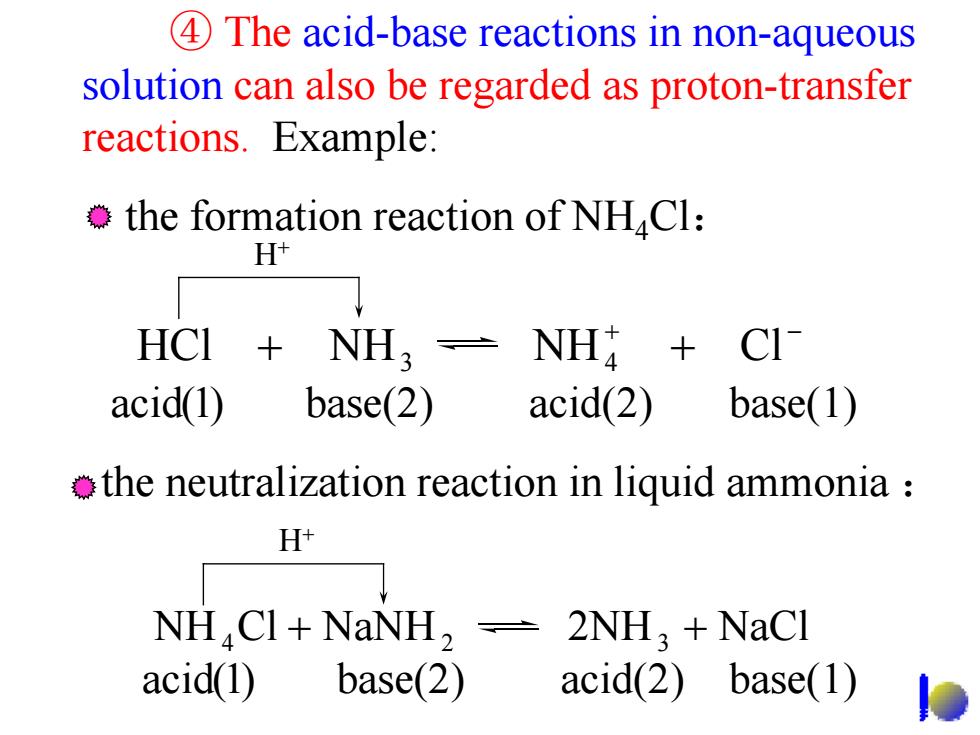
4 The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions.Example: the formation reaction of NHCl: H+ HCI NH NH CI acid(1) base(2) acid(2) base(1) the neutralization reaction in liquid ammonia H+ NH CI+NaNH,2NH+NaCl acid(1) base(2) acid(2) base(1)
④ The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions. Example: the formation reaction of NH4Cl: H+ the neutralization reaction in liquid ammonia : H+ + − HCl + NH NH + Cl 3 4 NH Cl NaNH 2NH NaCl 4 + 2 3 + acid(1) base(2) acid(2) base(1) acid(1) base(2) acid(2) base(1)
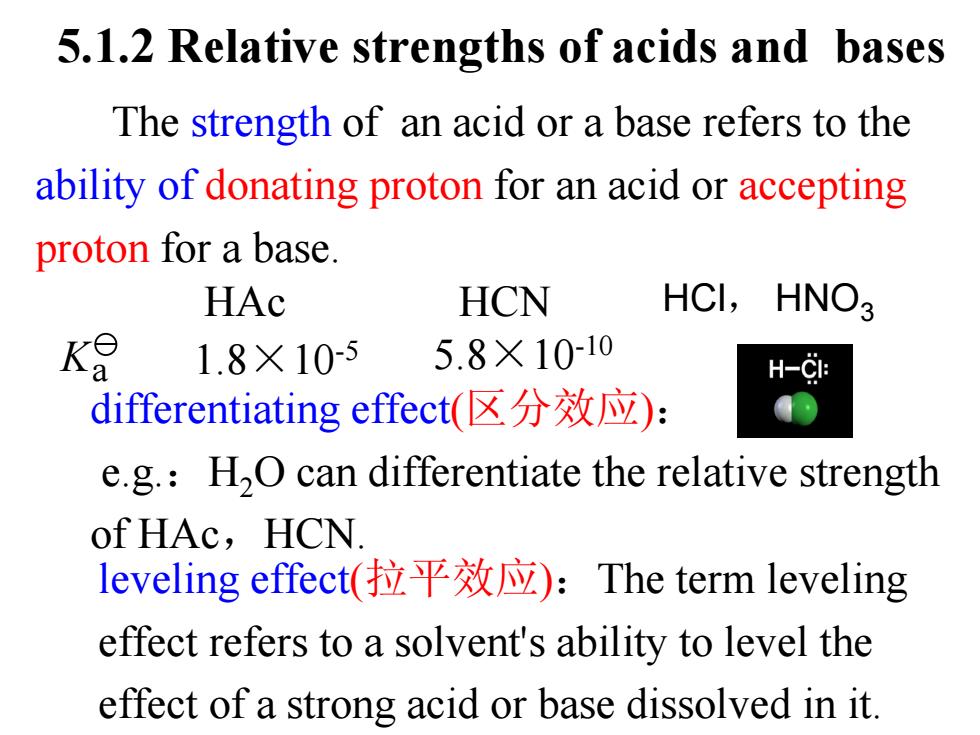
5.1.2 Relative strengths of acids and bases The strength of an acid or a base refers to the ability of donating proton for an acid or accepting proton for a base. HAc HCN HCI,HNO3 K 1.8×105 5.8×10-10 H-C1: differentiating effect((区分效应): e.g.:H2O can differentiate the relative strength of HAc,HCN. leveling effect(拉平效应):The term leveling effect refers to a solvent's ability to level the effect of a strong acid or base dissolved in it
HAc HCN differentiating effect(区分效应): e.g.:H2O can differentiate the relative strength of HAc,HCN. leveling effect(拉平效应):The term leveling effect refers to a solvent's ability to level the effect of a strong acid or base dissolved in it. The strength of an acid or a base refers to the ability of donating proton for an acid or accepting proton for a base. 5.1.2 Relative strengths of acids and bases 1.8×10 K -5 a 5.8×10-10 HCl, HNO3