
Chap 11 Coordination Compound Structures X 11.1 Configurations and Magnetism of Coordination Compounds X $11.2 Chemical Bonding Theory in Complexes
Chap 11 Coordination Compound Structures §11.2 Chemical Bonding Theory in Complexes §11.1 Configurations and Magnetism of Coordination Compounds
11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1.configurations 2.isomers 11.1.2 Magnetism of Coordination Compounds
11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1. configurations 2. isomers 11.1.2 Magnetism of Coordination Compounds
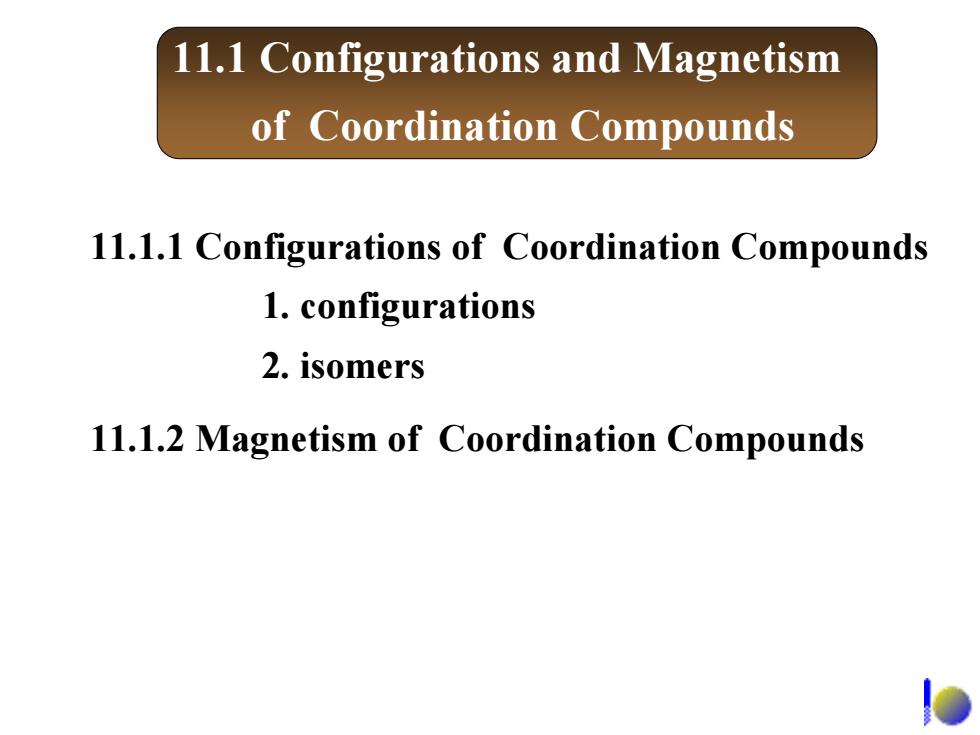
11.1.1 Configurations of coordination compounds 1.Geometric arrangements of atoms in a coordination compound are related to coordination number and ligand type 00 00-0 88 dodecahedral 正十二面体形 linear tetrahedral Square planer octahedral (8 vertex顶点) NH3 2+ 2+ AgNH3)克 H3N- NH3 H-N NH3 NH3 o
11.1.1 Configurations of coordination compounds 1. Geometric arrangements of atoms in a coordination compound are related to coordination number and ligand type linear Square planer tetrahedral octahedral dodecahedral 正十二面体形 (8 vertex 顶点 ) + 23 )Ag(NH
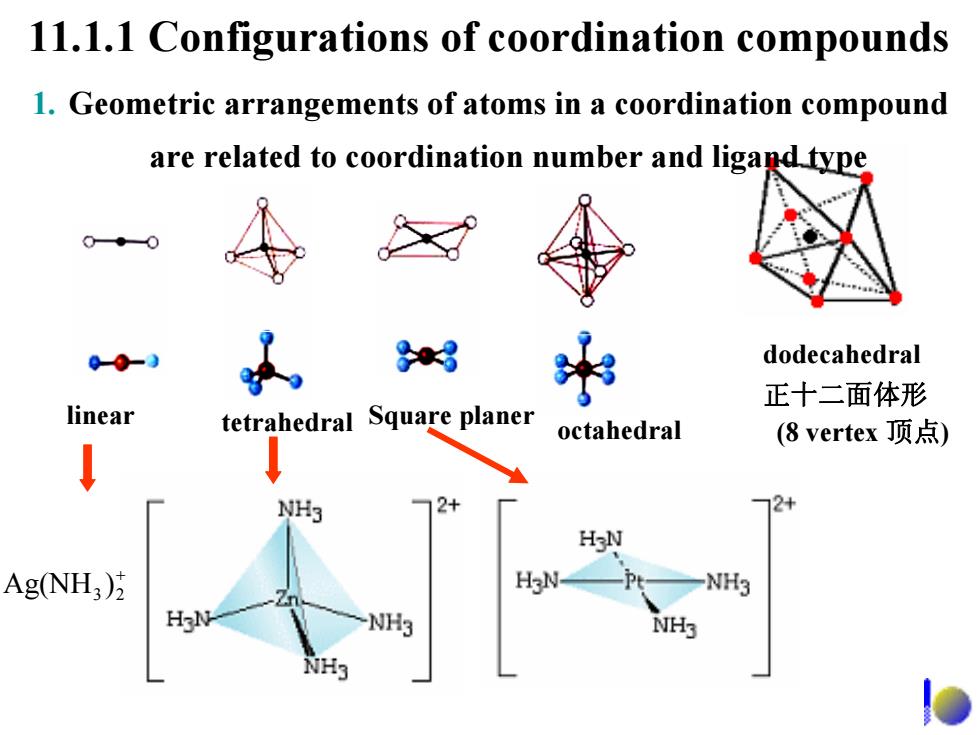
octahedron 八面体形 CoEDTA- Fe(CN) 0 0 HO-C-H2C CH,-C-OH HO-C-H2C N.CH.-CIN CH2-C-OH 0 0
O HO-C-H2C O HO-C-H2C N-CH2-CH2-N O CH2-C-OH O CH2-C-OH 乙二胺四乙酸 octahedron 八面体形 3− Fe(CN)6
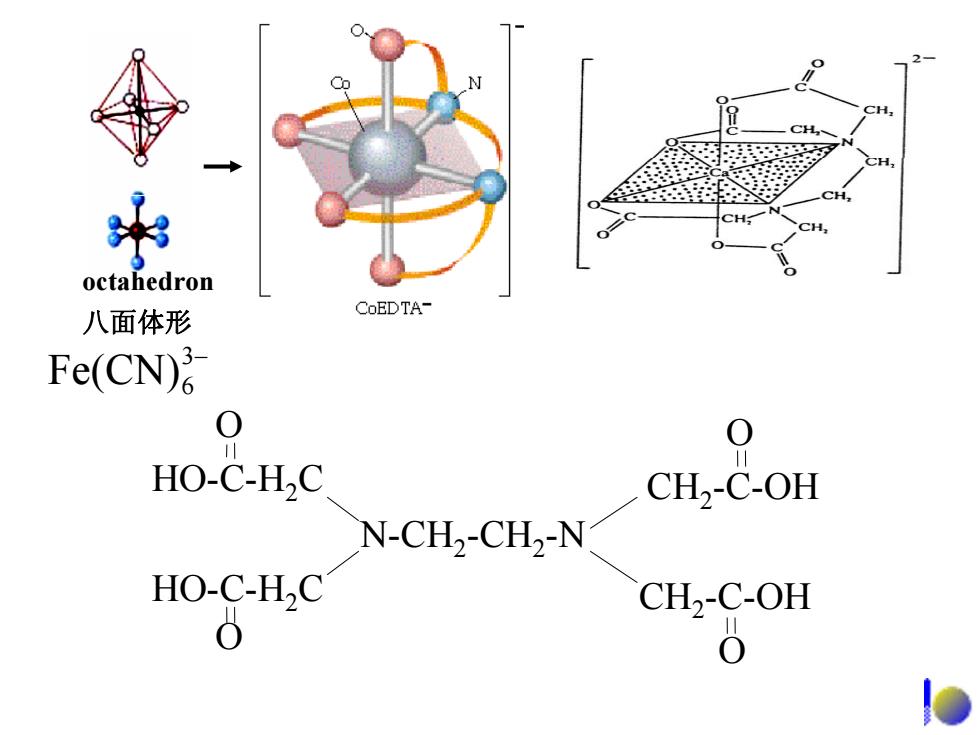
Hgl, 不N SbCI Square pyramidal Triangular 四方锥形 平面三角形 Triangle bipyramidal 三角双锥形 Fe(CO)s
Triangular 平面三角形 Square pyramidal 四方锥形 Triangle bipyramidal 三角双锥形 − HgI3 2− SbCl5 Fe(CO)5

83 Triangular Square pyramidal Square planer linear tetragonal octahedral Rules 1.In chemistry,a coordination complex or metal complex,is a structure consisting of Triangle bipyramidal a central atom or ion (usually metallic), bonded to a surrounding array of molecules or anions (ligands,complexing agents). 2.Ligands tend to stay away to have low energy and stability
linear Square planer octahedral Triangular Square pyramidal Triangle bipyramidal tetragonal • 1.In chemistry, a coordination complex or metal complex, is a structure consisting of a central atom or ion (usually metallic), bonded to a surrounding array of molecules or anions (ligands, complexing agents). • 2.Ligands tend to stay away to have low energy and stability Rules
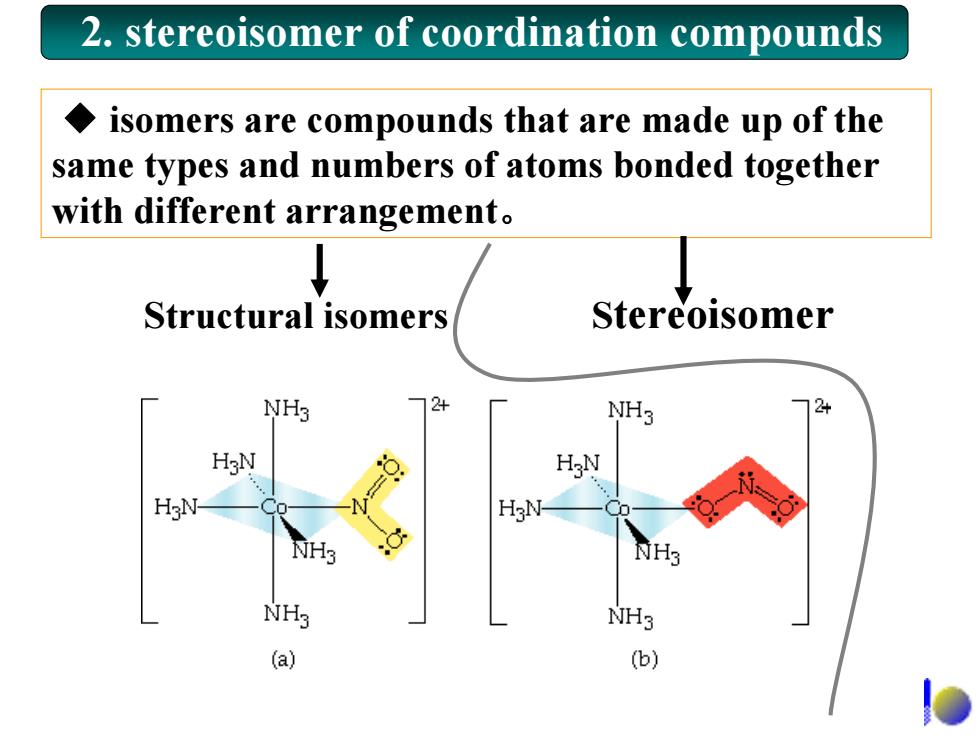
2.stereoisomer of coordination compounds isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement. Structural isomers Stereoisomer NH3 24 NH3 H N 1 的H3 NH3 NH3 (a) (b)
◆ isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement。 2. stereoisomer of coordination compounds Structural isomers Stereoisomer
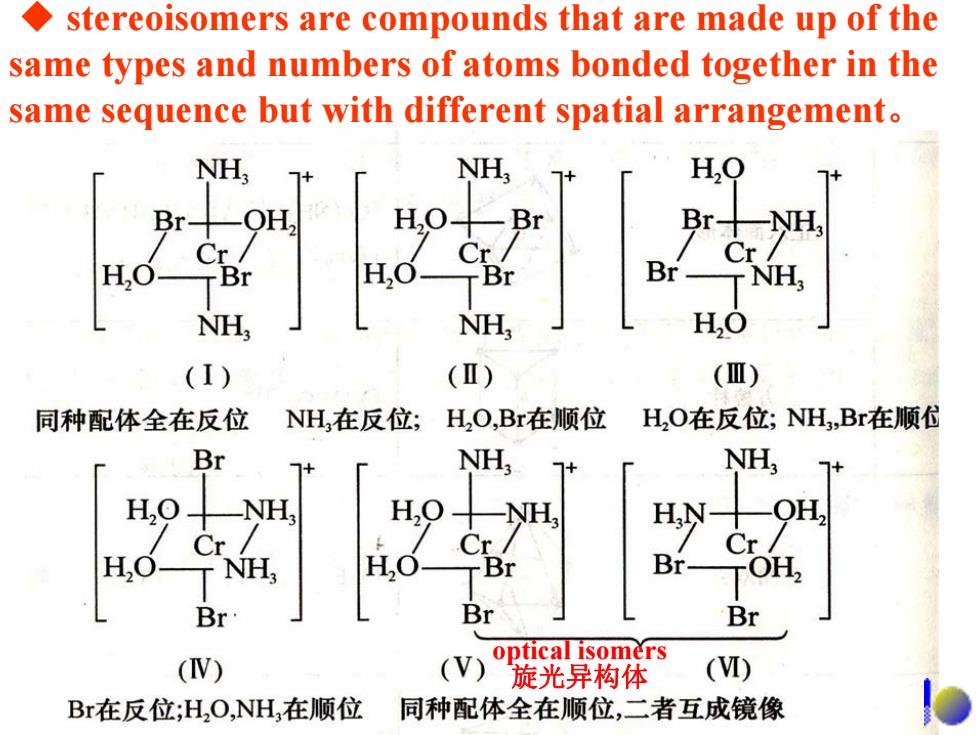
stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement. NH NH, H,O Br十 Br十 Cr Cr/ Cr/ H,C H,0 TBr Br-NH, NH, NH H,O (I) (Ⅱ) (Ⅲ) 同种配体全在反位 NH,在反位; H,O,Br在顺位 H,O在反位;NH,Br在顺 Br NH, NH, NH H, OH, Cr/ Cr/ Cr/ H,O- r NH, H,O Br- TOH, Br Br Br optical isomers (V) (V) 旋光异构体 (I) Br在反位;H,O,NH,在顺位 同种配体全在顺位,二者互成镜像
◆ stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement。 optical isomers 旋光异构体
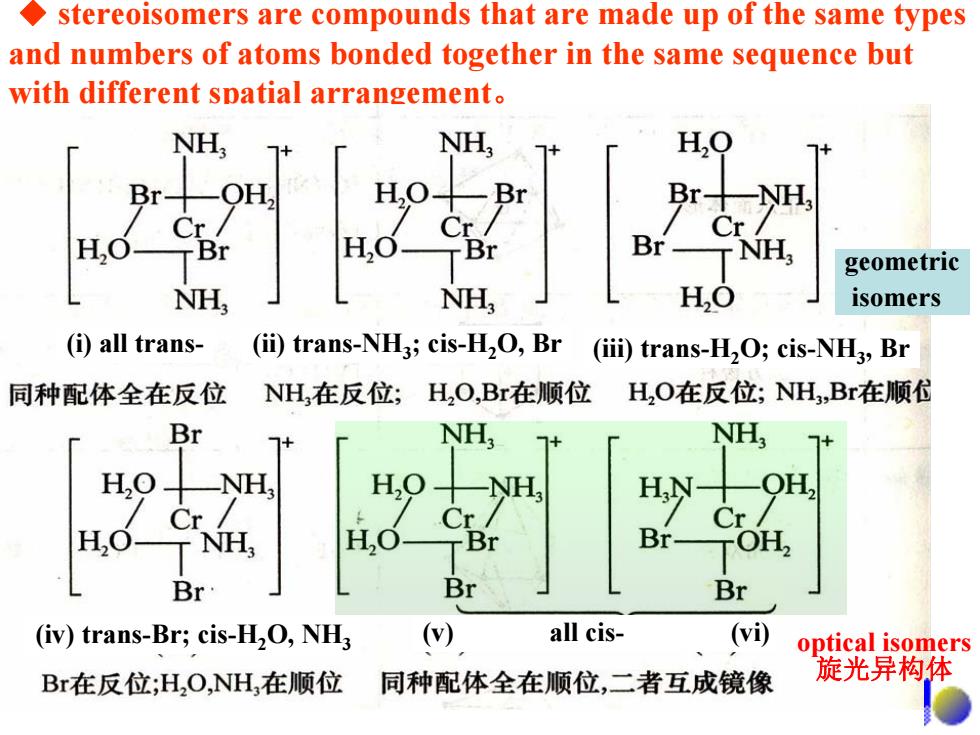
stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement. NH, NH, H,Q Br- H0十 Br Br十 NH Cr Cr H,O TBr Br- T NH geometric NH, NH H,O isomers (i)all trans- (ii)trans-NH3;cis-H2O,Br (iii)trans-H2O;cis-NH3,Br 同种配体全在反位 NH,在反位;H,O,Br在顺位 H,O在反位;NH,Br在顺付 Br NH; NH, H.O NH, HN- OH, Cr/ Cr Cr H,O-NH, H,0 TBr Br- TOH Br Br Br (iv)trans-Br;cis-H2O,NH3 () all cis- (vi) optical isomers Br在反位;H,O,NH,在顺位 同种配体全在顺位,二者互成镜像 旋光异构体
◆ stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement。 optical isomers 旋光异构体 geometric isomers (i) all trans- (ii) trans-NH3; cis-H2O, Br (iii) trans-H2O; cis-NH3, Br (iv) trans-Br; cis-H2O, NH3 (v) all cis- (vi)
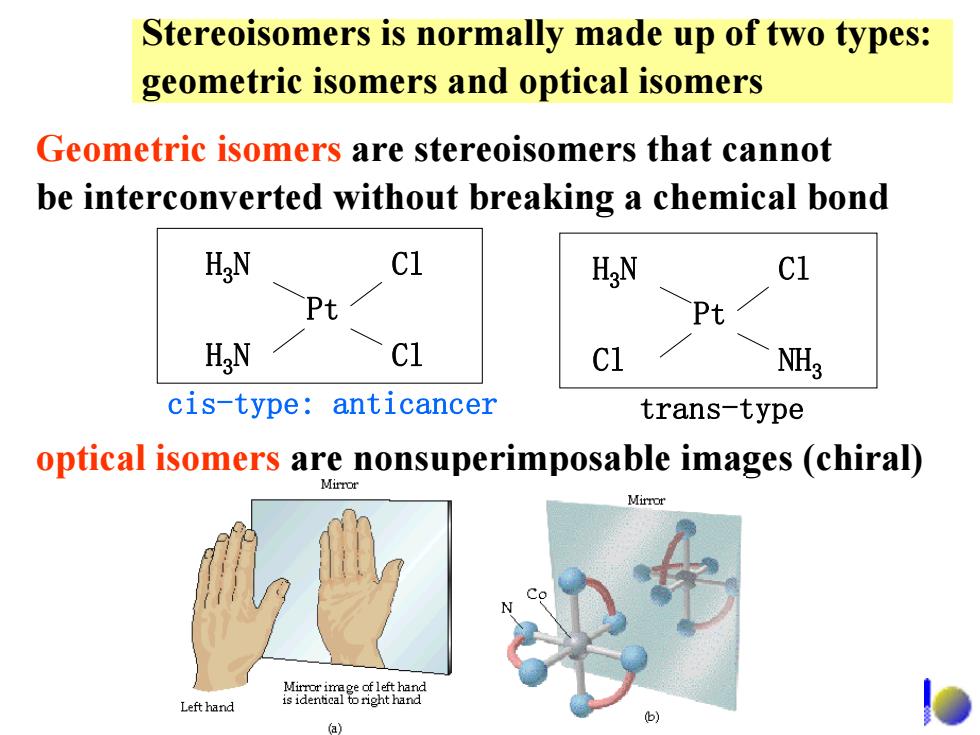
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond HaN C1 H N c1 Pt Pt HaN c1 NH cis-type:anticancer trans-type optical isomers are nonsuperimposable images (chiral) Mirror Left hand 01
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond optical isomers are nonsuperimposable images (chiral) H 3 N H 3 N Pt Cl Cl H 3 N Cl Pt Cl NH 3 cis-type: anticancer trans-type