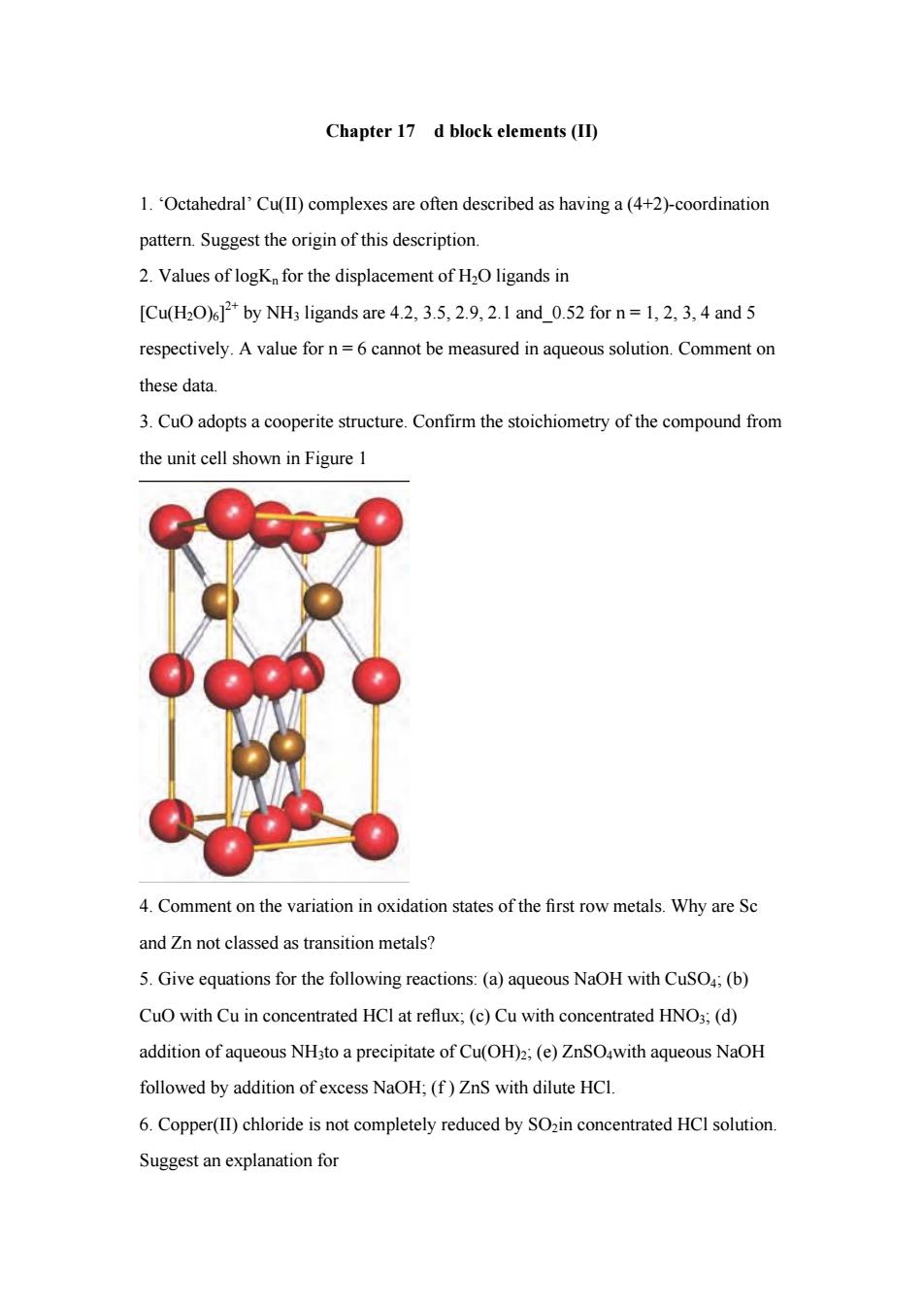
Chapter 17 d block elements(II) 1.Octahedral'Cu(ll)complexes are often described as having a(4+2)-coordination pattern.Suggest the origin of this description. 2.Values of logK for the displacement of H2O ligands in [Cu(H2O)by NH;ligands are 4.2.3.5,2.9.2.1 and_0.52 for n=1.2.3,4 and 5 respectively.A value forn=6cannot be measured in aqueous solution.Comment or these data. 3.Cu adopts a cooperite structure.Confirm the stoichiometry of the compound from the unit cell shown in Figure 1 4.Comment on the variation in oxidation states of the first row metals.Why are Sc and Zn not classed as transition metals? 5.Give equations for the following reactions:(a)aqueous NaOH with CuSO (b) CuO with Cu in concentrated HCI at reflux;(c)Cu with concentrated HNO3;(d) addition of aqueous NH3to a precipitate of Cu(OH)2;(e)ZnSO,with aqueous NaOH followed by addition of excess NaOH;(f)ZnS with dilute HCI. 6.Copper(II)chloride is not completely reduced by SOzin concentrated HCI solution Suggest an explanation for
Chapter 17 d block elements (II) 1. ‘Octahedral’ Cu(II) complexes are often described as having a (4+2)-coordination pattern. Suggest the origin of this description. 2. Values of logKn for the displacement of H2O ligands in [Cu(H2O)6] 2+ by NH3 ligands are 4.2, 3.5, 2.9, 2.1 and_0.52 for n = 1, 2, 3, 4 and 5 respectively. A value for n = 6 cannot be measured in aqueous solution. Comment on these data. 3. CuO adopts a cooperite structure. Confirm the stoichiometry of the compound from the unit cell shown in Figure 1 4. Comment on the variation in oxidation states of the first row metals. Why are Sc and Zn not classed as transition metals? 5. Give equations for the following reactions: (a) aqueous NaOH with CuSO4; (b) CuO with Cu in concentrated HCl at reflux; (c) Cu with concentrated HNO3; (d) addition of aqueous NH3to a precipitate of Cu(OH)2; (e) ZnSO4with aqueous NaOH followed by addition of excess NaOH; (f ) ZnS with dilute HCl. 6. Copper(II) chloride is not completely reduced by SO2in concentrated HCl solution. Suggest an explanation for

this observation and state how you would try to establish if the explanation is correct. 7.When the ligands do not sterically control the coordination geometry,do 4-coordinate complexes of(a)Pd(II),(b)Cu(I)and(c)Zn(II)prefer to be square planar or tetrahedral?Explain your answer.In the absence of crystallographic data how could you distinguish between a square planar or tetrahedral structure for a Ni(II) complex? 8.Give a brief account of the variation in properties of binary oxides of the first row d-block metals on going from Sc to Zn 9.When HS is passed into a solution of copper(I)sulfate acidified with H2SO copper(Isulfide precipitates.When concentrated HSOis heated with metallic Cu. the principal sulfur-containing product is SObut a residue of copper(I)sulfide is als formed.Account for these reactions
this observation and state how you would try to establish if the explanation is correct. 7. When the ligands do not sterically control the coordination geometry, do 4-coordinate complexes of (a) Pd(II), (b) Cu(I) and (c) Zn(II) prefer to be square planar or tetrahedral? Explain your answer. In the absence of crystallographic data, how could you distinguish between a square planar or tetrahedral structure for a Ni(II) complex? 8. Give a brief account of the variation in properties of binary oxides of the first row d-block metals on going from Sc to Zn. 9.When H2S is passed into a solution of copper(II) sulfate acidified with H2SO4, copper(II) sulfide precipitates. When concentrated H2SO4is heated with metallic Cu, the principal sulfur-containing product is SO2but a residue of copper(II) sulfide is also formed. Account for these reactions