
Part A.Answer all eight questions(5 marks each). 1. Describe what happens in an electron capture process,and how it is observed An inner shell electron is captured by the nucleus,where it combines with a proton to form a neutron.It is observed by the gamma radiation which is emitted immediately after the capture process. Indicate whether each of the following processes have positive or negative values of AH and AS: △(Indicate+or-)△S(Indicate+or-) H0g→Hg+⅓02g 2C102am+02g→2C103(am C8H18g+12.502g→8C02g+9 H2Og) Cooling H2(0 from200℃to150 SF6→SF6g Which has a smaller radius:a Kr atom or a Sr2ion?Why? The Srion has a smaller radius.Although the Kr atom and Srion are isoelectronic.the Sr ion has two more protons,making its effective nuclear charge greater,hence its radius smaller. Name the strongest intermolecular force in each of the following pairs of species Species Strongest Intermolecular Force Two CH4molecules Dispersion(London)
Part A. Answer all eight questions (5 marks each). 1. Describe what happens in an electron capture process, and how it is observed. An inner shell electron is captured by the nucleus, where it combines with a proton to form a neutron. It is observed by the gamma radiation which is emitted immediately after the capture process. 2. Indicate whether each of the following processes have positive or negative values of ΔH and ΔS: ΔH (Indicate + or -) ΔS (Indicate + or -) H2O(g) → H2(g) + ½ O2(g) + + 2 ClO2 – (aq) + O2(g) → 2 ClO3 – (aq) - - C8H18(g) + 12.5 O2(g) → 8 CO2(g) + 9 H2O(g) - + Cooling H2O(g) from 200o C to 150o C - - SF6(s) → SF6(g) + + 3. Which has a smaller radius: a Kr atom or a Sr+2 ion? Why? The Sr+2 ion has a smaller radius. Although the Kr atom and Sr+2 ion are isoelectronic, the Sr+2 ion has two more protons, making its effective nuclear charge greater, hence its radius smaller. 4. Name the strongest intermolecular force in each of the following pairs of species: Species Strongest Intermolecular Force Two CH4 molecules Dispersion (London)
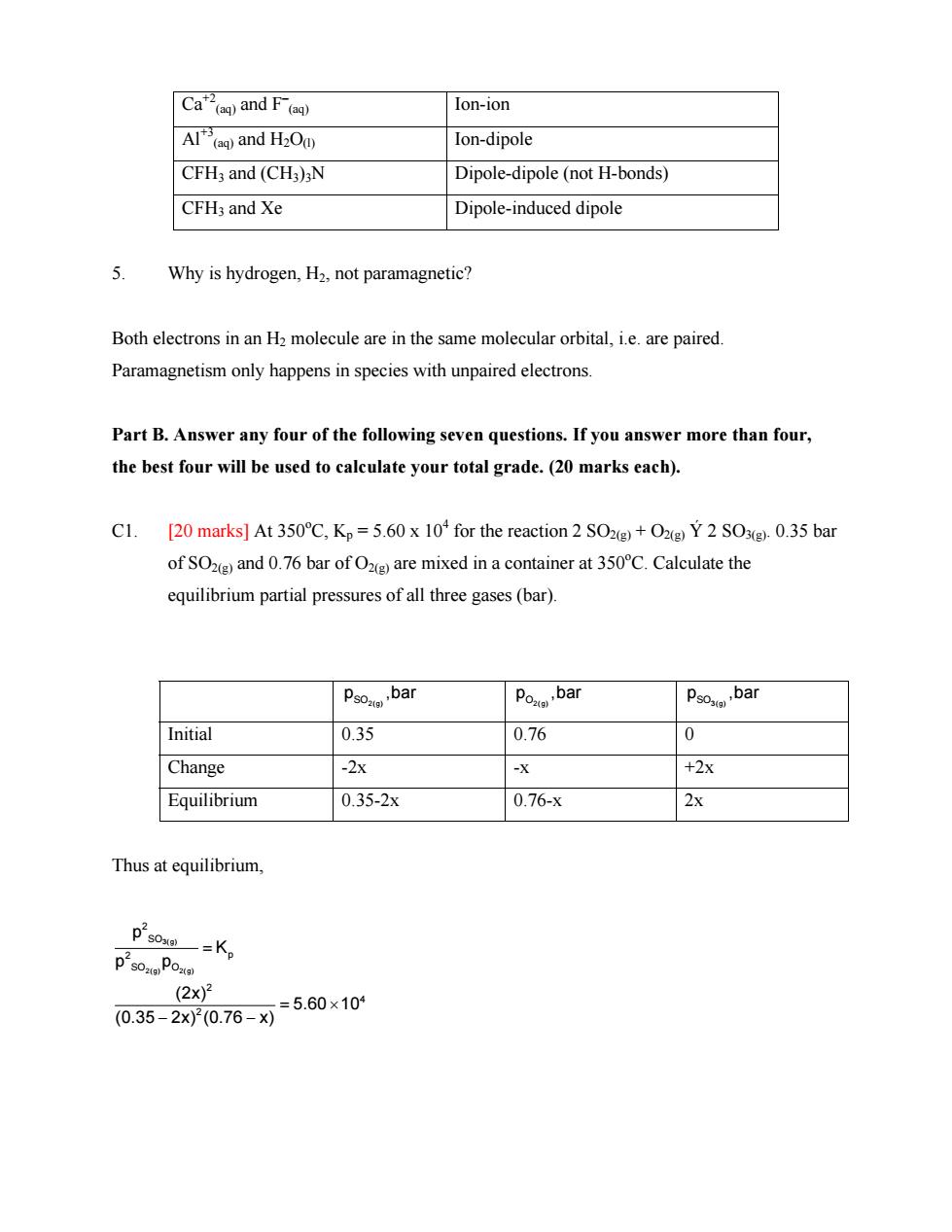
Ca”and F(e Ion-ion Al()and H2Od) Ion-dipole CFH3 and (CH3)3N Dipole-dipole(not H-bonds) CFH3 and Xe Dipole-induced dipole 5 Why is hydrogen,H2.not paramagnetic? Both electrons in an H2 molecule are in the same molecular orbital,i.e.are paired Paramagnetism only happens in species with unpaired electrons Part B.Answer any four of the following seven questions.If you answer more than four, the best four will be used to calculate your total grade.(20 marks each). [20 marks]At 350C.Kp=5.60 x 10 for the reaction 2 SO2+O Y 2 SOx(g).0.35 bar of SO(and 0.76 bar ofO are mixed in a container at 350C.Calculate the equilibrium partial pressures of all three gases(bar). Initial 0.35 0.76 0 Change -2x -X +2X Equilibrium 0.35-2x 0.76-x 2x Thus at equilibrium, ps0一=Kp ps0Pom (2x)2 0.35-2x00.76-X=5.60×10
Ca+2 (aq) and F– (aq) Ion-ion Al+3 (aq) and H2O(l) Ion-dipole CFH3 and (CH3)3N Dipole-dipole (not H-bonds) CFH3 and Xe Dipole-induced dipole 5. Why is hydrogen, H2, not paramagnetic? Both electrons in an H2 molecule are in the same molecular orbital, i.e. are paired. Paramagnetism only happens in species with unpaired electrons. Part B. Answer any four of the following seven questions. If you answer more than four, the best four will be used to calculate your total grade. (20 marks each). C1. [20 marks] At 350o C, Kp = 5.60 x 104 for the reaction 2 SO2(g) + O2(g) Ý 2 SO3(g). 0.35 bar of SO2(g) and 0.76 bar of O2(g) are mixed in a container at 350o C. Calculate the equilibrium partial pressures of all three gases (bar). SO2(g) p ,bar O2(g) p ,bar SO3(g) p ,bar Initial 0.35 0.76 0 Change -2x -x +2x Equilibrium 0.35-2x 0.76-x 2x Thus at equilibrium, 3(g) 2(g) 2(g) 2 SO 2 p SO O 2 4 2 p K p p (2x) 5.60 10 (0.35 2x) (0.76 x) = = × − −
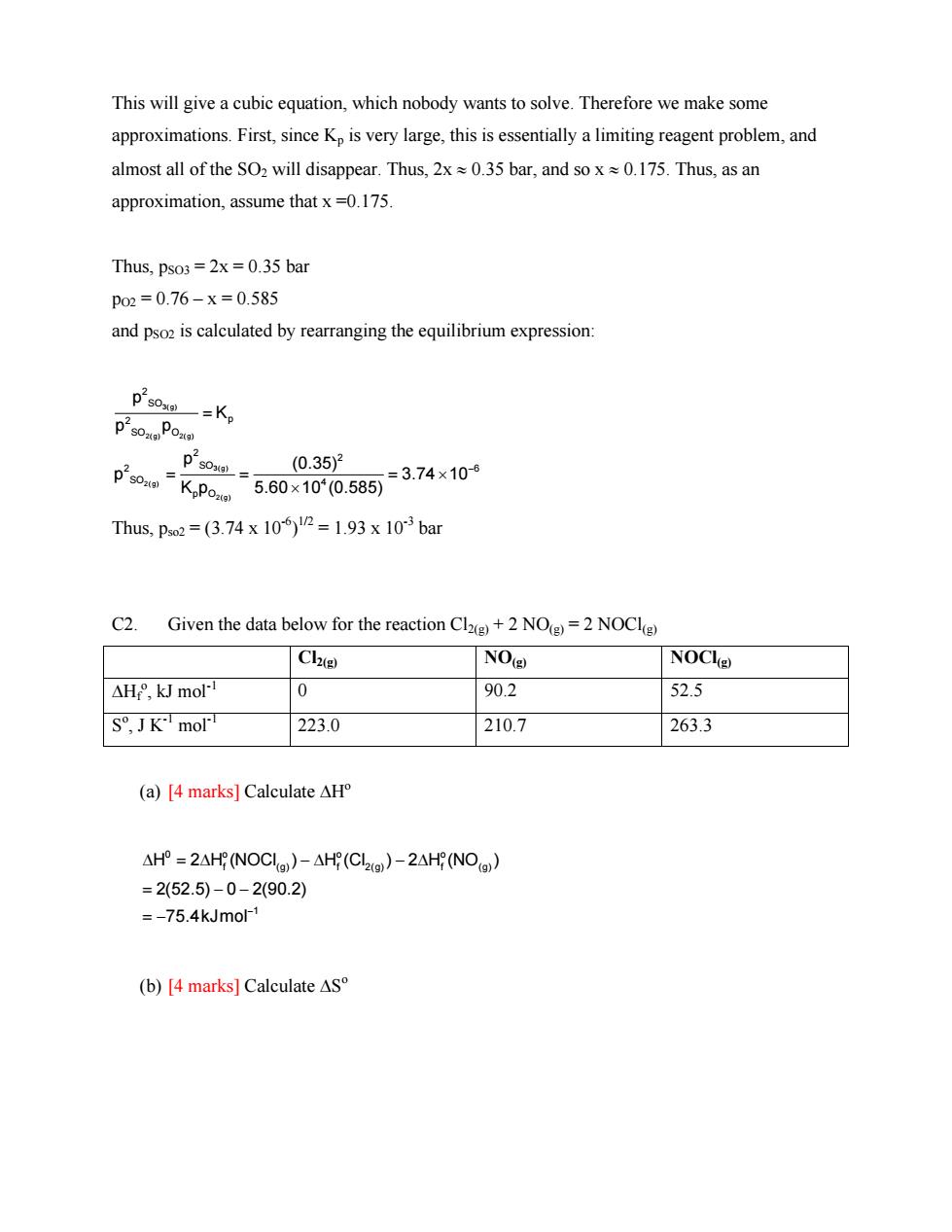
This will give a cubic equation,which nobody wants to solve.Therefore we make some approximations.First,since Kp is very large,this is essentially a limiting reagent problem,and almost all of the SO2 will disappear.Thus,2x0.35 bar,and so x0.175.Thus,as an approximation,assume that x=0.175. Thus,pso3=2x=0.35 bar po2=0.76-x=0.585 and pso2 is calculated by rearranging the equilibrium expression p2s0x一=K, p290Poao P'so K Po. 91 (0.35)2 5.60x100.5853.74x10 Thus,ps2=(3.74x10P=1.93x10-3ba C2. Given the data below for the reaction Cl+2 NO=2 NOCl) Clae NO( NOClo) △H,kJ mol" 0 90.2 52.5 S,JKmol 223.0 210.7 263.3 (a)[4 marks]Calculate AH △HP=2 AH(NOCI)-△H(CL2g)-2AH(NOa) =2(52.5)-0-2(90.2) =-75.4kJmo (b)[4 marks]Calculate AS
This will give a cubic equation, which nobody wants to solve. Therefore we make some approximations. First, since Kp is very large, this is essentially a limiting reagent problem, and almost all of the SO2 will disappear. Thus, 2x ≈ 0.35 bar, and so x ≈ 0.175. Thus, as an approximation, assume that x =0.175. Thus, pSO3 = 2x = 0.35 bar pO2 = 0.76 – x = 0.585 and pSO2 is calculated by rearranging the equilibrium expression: 3(g) 2(g) 2(g) 3(g) 2(g) 2(g) 2 SO 2 p SO O 2 2 2 SO 6 SO 4 p O p K p p p (0.35) p 3 K p 5.60 10 (0.585) .74 10− = = = =× × Thus, pso2 = (3.74 x 10-6) 1/2 = 1.93 x 10-3 bar C2. Given the data below for the reaction Cl2(g) + 2 NO(g) = 2 NOCl(g) Cl2(g) NO(g) NOCl(g) ΔHf o , kJ mol-1 0 90.2 52.5 So , J K-1 mol-1 223.0 210.7 263.3 (a) [4 marks] Calculate ΔHo 0 o o o f (g) f2(g) f( 1 H 2 H (NOCl ) H (Cl ) 2 H (NO 2(52.5) 0 2(90.2) 75.4kJmol− Δ = Δ −Δ − Δ = −− = − g) ) (b) [4 marks] Calculate ΔSo

AHP=2S(NOClo)-S°(Ca)-2S(NOa) =2(263.3)-223.0-2(210.7) =-117.8JK-mor (c)[4 marks]Calculate AG at 25C AGO AHO-TASO =-75400Jmor1-(25+273K(-117.8 JK-'mol') =-40296JK-mo =-40.3kJmo (d)[4 marks]Calculate the value of Kp at 50C e 40300Jmo1 =ex08314JKm0r50+273K =exp(15.0) =3.29×10 (e)[4 marks]Calculate K at 50C K。=K.RT)” =3.29×10(8.314JK-mor'(50+273)K)- =1225 C3.(a)Use VSEPR theory to predict the shapes of (i)[5 marks]PCl" 5+(4 x 7)-1=32 electrons
) −1 0 0 0 0 (g) 2(g) (g) 1 1 H 2S (NOCl ) S (Cl ) 2S (NO 2(263.3) 223.0 2(210.7) 117.8JK mol − − Δ= − − = −− = − (c) [4 marks] Calculate ΔGo at 25o C 00 0 1 1 1 1 1 G H TS 75400Jmol (25 273)K( 117.8JK mol ) 40296JK mol 40.3kJmol − − − − − Δ =Δ − Δ = − − + − = − = − (d) [4 marks] Calculate the value of Kp at 50o C 0 p 1 1 1 6 G K exp RT 40300Jmol exp 8.314JK mol (50 273)K exp(15.0) 3.29 10 − − − ⎛ ⎞ −Δ = ⎜ ⎟ ⎝ ⎠ ⎛ ⎞ = ⎜ ⎟ ⎝ ⎠ + = = × (e) [4 marks] Calculate Kc at 50o C n p c 6 11 K K (RT) 3.29 10 (8.314JK mol (50 273)K) 1225 Δ − − − = = × + = 1 C3. (a) Use VSEPR theory to predict the shapes of (i) [5 marks] PCl4 + 5 + (4 x 7) – 1 = 32 electrons

Arranging the four Cl atoms around the P atom,connecting them with single bonds and completing the octets on the Cl atoms uses all 32 electrons.The molecule is thus of the form AX,and must be tetrahedral. (ii)[5 marks]PCls 5+(5 x 7)+1=41 electrons Arranging the five Cl atoms around the Patom,connecting them with single bonds and completing the octets on the Cl atoms uses 40 electrons.The last electron goes on the P atom as a single electron.The molecule is thus of the form AXsE and must be square pyramidal (b)[10 marks]How much energy (in kJ mol)is required to ionize H atoms whose electrons are initially in the m=2 shell?The Rydberg constant,R,is0.01097nm 品剖 In this case,the electron is beginning at m=2 and being removed from the atom,i.e.n=. Thus, 是R安剖 =0.25R =0.25(0.01097nm-) =0.00274nm- 1 1=0.0274nm-365nm E=hu-he 6.63×1034Js(3.00×10°ms) 365×10-m =5.45x10-1J(perphoton) ×6.02×1023mor1=3.28×105Jmo =32.8kJmol-
Arranging the four Cl atoms around the P atom, connecting them with single bonds and completing the octets on the Cl atoms uses all 32 electrons. The molecule is thus of the form AX4 and must be tetrahedral. (ii) [5 marks] PCl5 – 5 + (5 x 7) + 1 = 41 electrons Arranging the five Cl atoms around the P atom, connecting them with single bonds and completing the octets on the Cl atoms uses 40 electrons. The last electron goes on the P atom as a single electron. The molecule is thus of the form AX5E and must be square pyramidal. (b) [10 marks] How much energy (in kJ mol-1) is required to ionize H atoms whose electrons are initially in the m = 2 shell? The Rydberg constant, R, is 0.01097 nm-1. 2 2 1 11 R m n ⎛ ⎞ = − ⎜ ⎟ λ ⎝ ⎠ In this case, the electron is beginning at m = 2 and being removed from the atom, i.e. n = ∞. Thus, 2 2 1 1 1 34 8 1 9 19 23 1 5 1 1 1 11 R 2 0.25R 0.25(0.01097nm ) 0.00274nm 1 365nm 0.00274nm hc E h 6.63 10 Js(3.00 10 ms ) 365 10 m 5.45 10 J(per photon) 6.02 10 mol 3.28 10 Jmol 32.8kJmol − − − − − − − − − − ⎛ ⎞ = − ⎜ ⎟ λ ∞ ⎝ ⎠ = = = λ = = = υ= λ × × = × = × ×× = × =

C4.[8 marks](a)One of the few electrochemical processes that produces an organic compound is used by the Monsanto Company to produce 1,4-dicyanobutane according to the reduction reaction: CH2CHCN +2 H*+2 e>NC-(CH2)4-CN What current(in Amperes)must be used to produce 150 kg of the product per hour? 150 kg/hx 1000 g/kgx(1 mol product/108 g product)x(2 mol e/mol product) x96000C/mole=2.67x103Ch =7.41x104Cs=74100A [12 marks](b)Balance the following REDOX reaction in acidic solution: MnO4aa+CIam→Mn”am+Cl2e Oxidation CTag)→Cl2g 2CT(am→Cl2g 2CT(am→Cl2g+2e Reduction MnO4am→Mn2am MnO4e→Mn”em+4H0U MnO ()+8 H'(ag)>Mn2()+4 H2Od) MnO(0Q+8 H'0)+5 e>Mn"+4 H2O0) Cross Multiply 52CT(am→Cl2g+2e) 2(Mn04a+8fam+5e→Mn2a+4H20d)
C4. [8 marks] (a) One of the few electrochemical processes that produces an organic compound is used by the Monsanto Company to produce 1,4-dicyanobutane according to the reduction reaction: CH2CHCN + 2 H+ + 2 e- → NC-(CH2)4-CN What current (in Amperes) must be used to produce 150 kg of the product per hour? 150 kg/h x 1000 g/kg x (1 mol product / 108 g product) x (2 mol e- / mol product) x 96000 C/mol e- = 2.67 x 108 C/h = 7.41 x 104 C/s = 74100 A [12 marks] (b) Balance the following REDOX reaction in acidic solution: MnO4 – (aq) + Cl– (aq) → Mn+2 (aq) + Cl2(g) Oxidation Cl– (aq) → Cl2(g) 2 Cl– (aq) → Cl2(g) 2 Cl– (aq) → Cl2(g) + 2 e– Reduction MnO4 – (aq) → Mn+2 (aq) MnO4 – (aq) → Mn+2 (aq) + 4 H2O(l) MnO4 – (aq) + 8 H+ (aq) → Mn+2 (aq) + 4 H2O(l) MnO4 – (aq) + 8 H+ (aq) + 5 e– → Mn+2 (aq) + 4 H2O(l) Cross Multiply 5(2 Cl– (aq) → Cl2(g) + 2 e– ) 2(MnO4 – (aq) + 8 H+ (aq) + 5 e– → Mn+2 (aq) + 4 H2O(l))

10Cam→5Cl2g+10e 2 MnO4 (ag)+16 H'(ag)+10 e>2 Mn(ag)+8 H2Od) Add 10 Cl(ag)+2 MnO4 (ag)+16 H(ag)>5 Cl2(g)+2 Mn"(ag)+8 H2O) C5.(a)[8 marks]An aqueous solution of a detergent at a concentration of 1.00gL has an osmotic pressure of 64.8 mm Hg at 25.0C.Calculate the molecular weight of the detergent. 64.8mmHa I760mmHg/atm =0.0853atm II=MRT 0.0853atm =0.082 LatmK 'mo'(25+273r =0.00349mo1L-1 The molecular weight is therefore 0.00349mol=286.6gmor, 1.00gL (b)[8 marks]Calculate the molality of a solution that is 30.0%by weight HO The density of the solution is 1.11gmL Assume 1 L of the solution.This has a mass of 1,000 mL x 1.11 g mL=1110g The mass of H2O2 in this solution is 30.0%(1110 g)=333 g Moles of H2O2=333 g/34.0 g/mol =9.79 mol Mass of water =1110g-333 g=777g
10 Cl– (aq) → 5 Cl2(g) + 10 e– 2 MnO4 – (aq) + 16 H+ (aq) + 10 e– → 2 Mn+2 (aq) + 8 H2O(l) Add 10 Cl– (aq) + 2 MnO4 – (aq) + 16 H+ (aq) → 5 Cl2(g) + 2 Mn+2 (aq) + 8 H2O(l) C5. (a) [8 marks] An aqueous solution of a detergent at a concentration of 1.00 g L-1 has an osmotic pressure of 64.8 mm Hg at 25.0o C. Calculate the molecular weight of the detergent. 1 1 1 64.8mmHg 0.0853atm 760mmHg / atm MRT M RT 0.0853atm 0.082LatmK mol (25 273)K 0.00349molL − − − Π = = Π = Π = = + = The molecular weight is therefore 1 1 1 1.00gL 286.6gmol 0.00349molL − − − = (b) [8 marks] Calculate the molality of a solution that is 30.0% by weight H2O2(aq). The density of the solution is 1.11 g mL-1. Assume 1 L of the solution. This has a mass of 1,000 mL x 1.11 g mL-1 = 1110 g The mass of H2O2 in this solution is 30.0%(1110 g) = 333 g Moles of H2O2 = 333 g / 34.0 g/mol = 9.79 mol Mass of water = 1110 g – 333 g = 777 g

Molality= molsolute 9.79 kgsolvent0.777-12.6m (c)[4 marks]Calculate the boiling point of a 5 m aqueous solution of ethanol,C2HOH. For water,Ko=0.51 C kg mol" △T。=K,m =0.51Ckgmol-'(5.0molkg') =2.55℃ Thus,T。=100.00°C+2.55°C =102.55C C6.(a)[10 marks]Calculate the pH of an aqueous solution that is 2.5 M triethylamine.(C2Hs)N K for this compound is 5.6x10 (C2Hs)3N+H2O Y(C2H5)3NH)+OHa) (C2Hs)3N (C2Hs)3NH'(a)OH9 Initial 2.5 0 Change -X +X +X Equilibrium 2.5-x 2器2x=5610 25*56×10 x2≈2.5(5.6×104) x2=1.4×10-3 ×=0.0374 Thus,[OH(a】=x=0.0374 pOH=-logIo[OH(ag]=-logIo(0.0374)=1.43
molsolute 9.79 Molality 12.6m kgsolvent 0.777 = == (c) [4 marks] Calculate the boiling point of a 5 m aqueous solution of ethanol, C2H5OH. For water, Kb = 0.51 o C kg mol-1 b b o 1 o o o b o T Km 0.51 Ckgmol (5.0molkg ) 2.55 C Thus,T 100.00 C 2.55 C 102.55 C − − Δ = = = = + = 1 C6. (a) [10 marks] Calculate the pH of an aqueous solution that is 2.5 M triethylamine, (C2H5)3N. Kb for this compound is 5.6 x 10-4 (C2H5)3N + H2O(l) Ý (C2H5)3NH+ (aq) + OH- (aq) (C2H5)3N (C2H5)3NH+ (aq) OH- (aq) Initial 2.5 0 0 Change -x +x +x Equilibrium 2.5-x x x 4 2 4 2 4 2 3 x(x) 5.6 10 2.5 x x 5.6 10 2.5 x 2.5(5.6 10 x 1.4 10 x 0.0374 − − − − = × − ≈ × ≈ × = × = ) Thus, [OH- (aq)] = x = 0.0374 pOH = -log10[OH- (aq)] = -log10(0.0374) = 1.43

pH=14-p0H=14-1.43=12.6 (b)[10 marks]Calculate the pH of an aqueous solution that is 2.5 M triethylamine and 1.2 M triethylamine hydrochloride,(C2Hs)3NHCla) The two species constitute a basic buffer system the triethylamine is the base and the triethylamine hydrochloride is a salt of the base. pK=14-pK=14-log10(Kb)=14-log10(5.6x104=14-3.25=10.75 HK.og) =1075-692) =11.1 C7.20 marks]Gold crystallizes in a face centred cubic unit cell.The radius of a gold atom is 144 pm.Calculate the density of gold metal (in g cm). In a face centred cubic unit cell,there are 4 atoms in total. Thus,the unit cell mass=4 x mass of one gold atom=4(197 g/mol)/(6.02 x 1023 mol) =1.31x10-21g In a face-centred cubic unit cell,the diagonal of one face=4r.Thus,(4r)=1+12(where l is the edge length of the unit cell) thus.16r2=212,or2=8r2 or1=(8r22 =(8(1.44x10cm)9 =4.07x103cm
pH = 14 - pOH = 14 – 1.43 = 12.6 (b) [10 marks] Calculate the pH of an aqueous solution that is 2.5 M triethylamine and 1.2 M triethylamine hydrochloride, (C2H5)3NHCl(aq) The two species constitute a basic buffer system the triethylamine is the base and the triethylamine hydrochloride is a salt of the base. pKa = 14 – pKb = 14 – log10(Kb) = 14 – log10(5.6 x 10-4) = 14 – 3.25 = 10.75 a 10 10 [acid] pH pK log [base] 1.2 10.75 log 2.5 11.1 ⎛ ⎞ = − ⎜ ⎟ ⎝ ⎠ ⎛ ⎞ = − ⎜ ⎟ ⎝ ⎠ = C7. [20 marks] Gold crystallizes in a face centred cubic unit cell. The radius of a gold atom is 144 pm. Calculate the density of gold metal (in g cm-3). In a face centred cubic unit cell, there are 4 atoms in total. Thus, the unit cell mass = 4 x mass of one gold atom = 4(197 g/mol)/(6.02 x 1023 mol-1) = 1.31 x 10-21 g. In a face-centred cubic unit cell, the diagonal of one face = 4r. Thus, (4r)2 = l2 + l2 (where l is the edge length of the unit cell) thus, 16 r2 = 2 l2 , or l2 = 8r2 , or l = (8r2 ) 1/2 = (8(1.44 x 10-8cm)2 ) 1/2 = 4.07 x 10-8 cm

Volume of the unit cell is V= =(4.07x108cm)3 =6.76x10r23cm Density=mass/volume =1.31x1021g/6.76x10r23cm =19.4gcm
Volume of the unit cell is V = l3 = (4.07 x 10-8 cm)3 = 6.76 x 10-23 cm3 Density = mass/volume =1.31 x 10-21 g / 6.76 x 10-23 cm3 = 19.4 g cm-3