Chapter 3 Chemical kinetics 3.1 Brief introduction of chemical reaction rates 3.2 Brief introduction of effect of concentration of reactants on reaction rate-the rate expression 3.3 Brief introduction of effect of temperature on reaction rate-Arrhenius equation 3.4 Brief introduction of theory of reaction rate and reaction mechanisms 3.5 Brief introduction of catalyst and catalysis
Chapter 3 Chemical kinetics 3.1 Brief introduction of chemical reaction rates 3.2 Brief introduction of effect of concentration of reactants on reaction rate —the rate expression 3.3 Brief introduction of effect of temperature on reaction rate—Arrhenius equation 3.4 Brief introduction of theory of reaction rate and reaction mechanisms 3.5 Brief introduction of catalyst and catalysis

3.1 Brief Introduction of chemical reaction rates 3.1.1 Average rates and instantaneous rates of chemical reactions
§3.1 Brief Introduction of chemical reaction rates 3.1.1 Average rates and instantaneous rates of chemical reactions
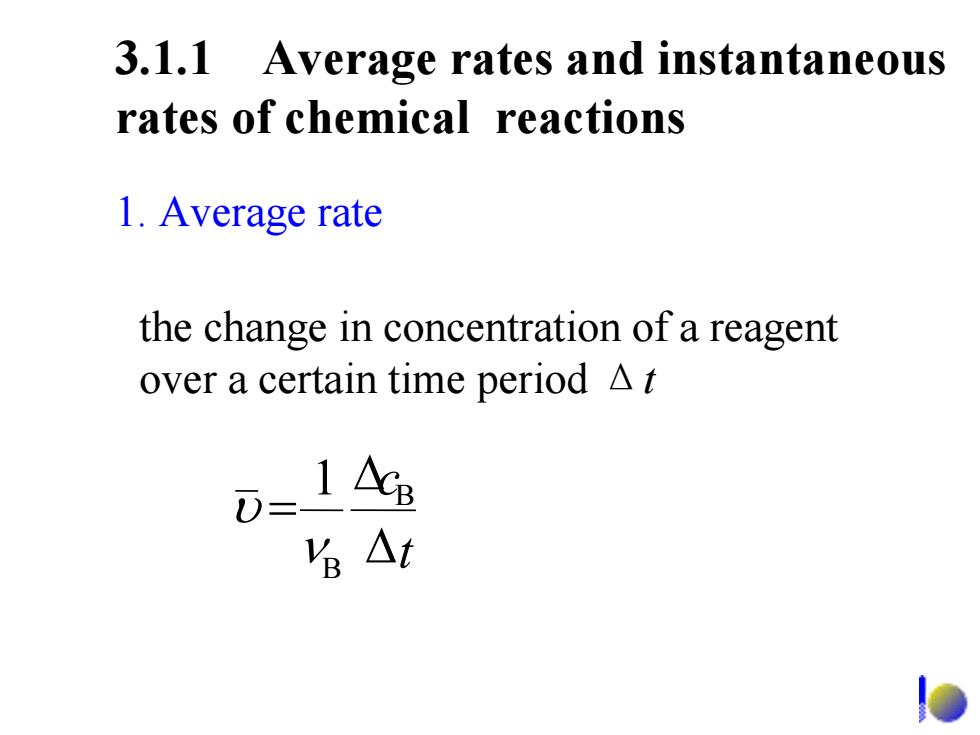
3.1.1 Average rates and instantaneous rates of chemical reactions 1.Average rate the change in concentration of a reagent over a certain time period A t 1 AcB 名△t
3.1.1 Average rates and instantaneous rates of chemical reactions 1. Average rate the change in concentration of a reagent over a certain time period Δ t 1 B B Δ Δ = t c ν υ
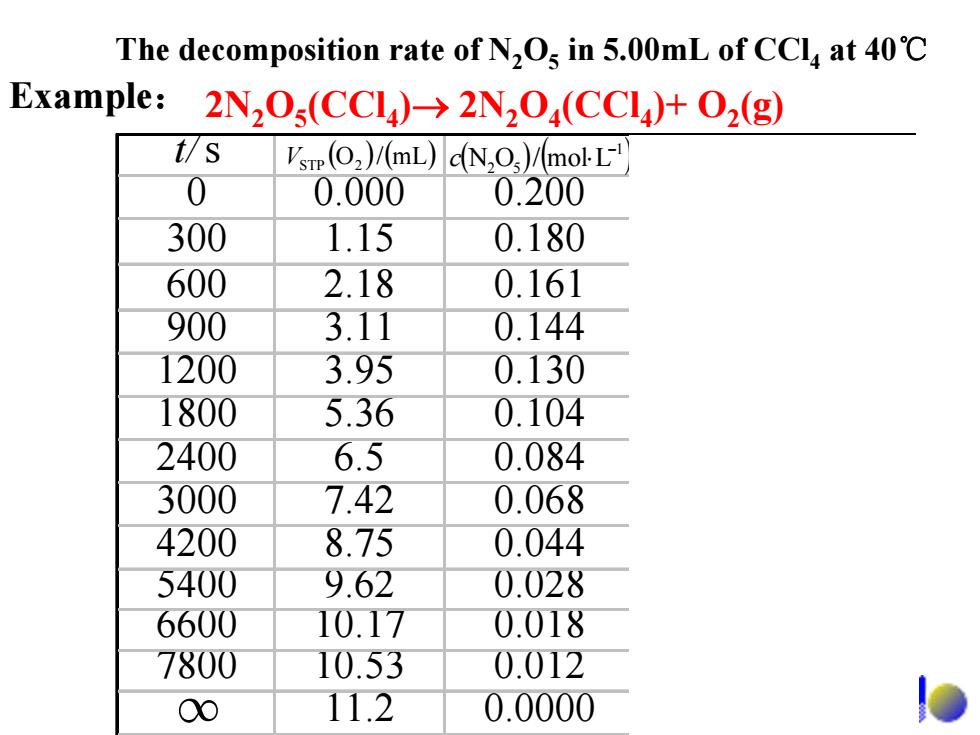
The decomposition rate of N,Os in 5.00mL of CCla at 40C Example:2N,O(CCI)>2N2O(CCI)+O2(g) t/S Vsrp(O2)/(mL)dN,O)/molL 0 0.000 0.200 300 1.15 0.180 600 2.18 0.161 900 3.11 0.144 1200 3.95 0.130 1800 5.36 0.104 2400 6.5 0.084 3000 7.42 0.068 4200 8.75 0.044 5400 9.62 0.028 6600 10.17 0.018 7800 10.53 0.012 00 11.2 0.0000
t/s 0 0.000 0.200 300 1.15 0.180 600 2.18 0.161 900 3.11 0.144 1200 3.95 0.130 1800 5.36 0.104 2400 6.5 0.084 3000 7.42 0.068 4200 8.75 0.044 5400 9.62 0.028 6600 10.17 0.018 7800 10.53 0.012 11.2 0.0000 ( ) (mL/O ) V 2STP ( ) ( )1 52 Lmol/ON − c ⋅ ( )11 sLmol/ −− υ ⋅⋅ 5 1029.7 − × 5 1046.6 − × 5 1080.5 − × 5 1021.5 − × 5 1069.4 − × 5 1079.3 − × 5 1004.3 − × 5 1003.1 − × 5 1044.2 − × 5 1059.1 − × ∞ The decomposition rate of N2O5 in 5.00mL of CCl4 at 40℃ 2N2O5(CCl4)→ 2N2O4(CCl4)+ O2 Example: (g)

2N205(CC14)2N204(CC14)+O2(g) t1=0s c1N205)=0.200molL1 t2=300s c2N20s)=0.180molL1 D=-(0.1800,200mol-L=33×109mol-L2-s 2×300s 2.Instantaneous rate The instantaneous rate of a reaction is the limit of the average reaction rate
t1= 0 s c1(N2O5) = 0.200 mol·L-1 t2=300 s c2(N2O5) = 0.180 mol·L-1 5 1-1- -1 sLmol103.3 s3002 Lmol ⋅⋅×= × (0.180− − 0.200) ⋅ = − υ 2. Instantaneous rate The instantaneous rate of a reaction is the limit of the average reaction rate. 2N2O4 (CCl4) + O2 2N (g) 2O5(CCl4)

3.2 Brief introduction of effect of concentration of reactants on reaction rate -the rate expression -3.2.1 The rate expression
§3.2 Brief introduction of effect of concentration of reactants on reaction rate —the rate expression 3.2.1 The rate expression
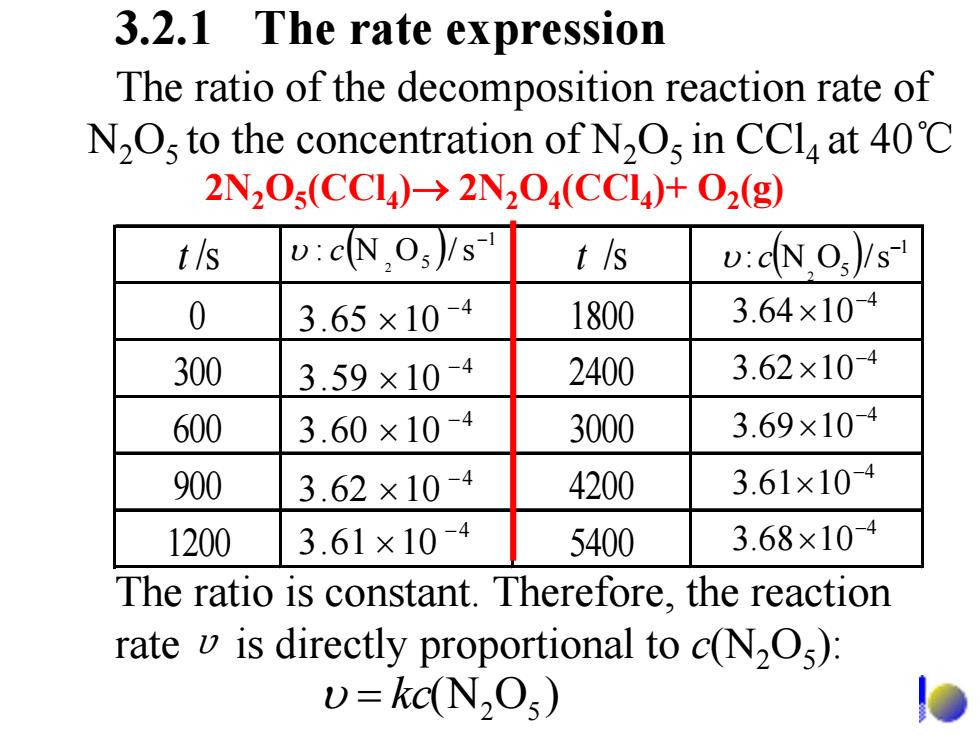
3.2.1 The rate expression The ratio of the decomposition reaction rate of N2Os to the concentration of N2Os in CCla at 40C 2N20s(CC4)-→2N204(CCl4)+O2(g) t/s v:cNOs)/s t/s v:dNO)/s- 0 3.65×10-4 1800 3.64×104 300 3.59×104 2400 3.62×104 600 3.60×10-4 3000 3.69×104 900 3.62×10-4 4200 3.61×104 1200 3.61×10-4 5400 3.68×104 The ratio is constant.Therefore,the reaction rate v is directly proportional to c(N2O): v=kc(N2Os)
The ratio of the decomposition reaction rate of N 2 O5 to the concentration of N 2 O5 in CCl4 at 40 ℃ t /s t /s 0 1800 300 2400 600 3000 900 4200 1200 5400 ( ) 1 5 s/ON: 2 − υ c 4 1065.3 − × 4 1060.3 − × 4 1062.3 − × 4 1061.3 − × 4 1059.3 − × 4 1069.3 − × 4 1062.3 − × 4 1064.3 − × 4 1068.3 − × 4 1061.3 − × ( ) 1 5 s/ON: 2 − υ c 3.2.1 The rate expression The ratio is constant. Therefore, the reaction rate υ is directly proportional to c(N 2 O 5): )ON( 52 υ = kc 2N 2 O 5(CCl 4 ) → 2N 2 O 4(CCl 4)+ O 2(g)
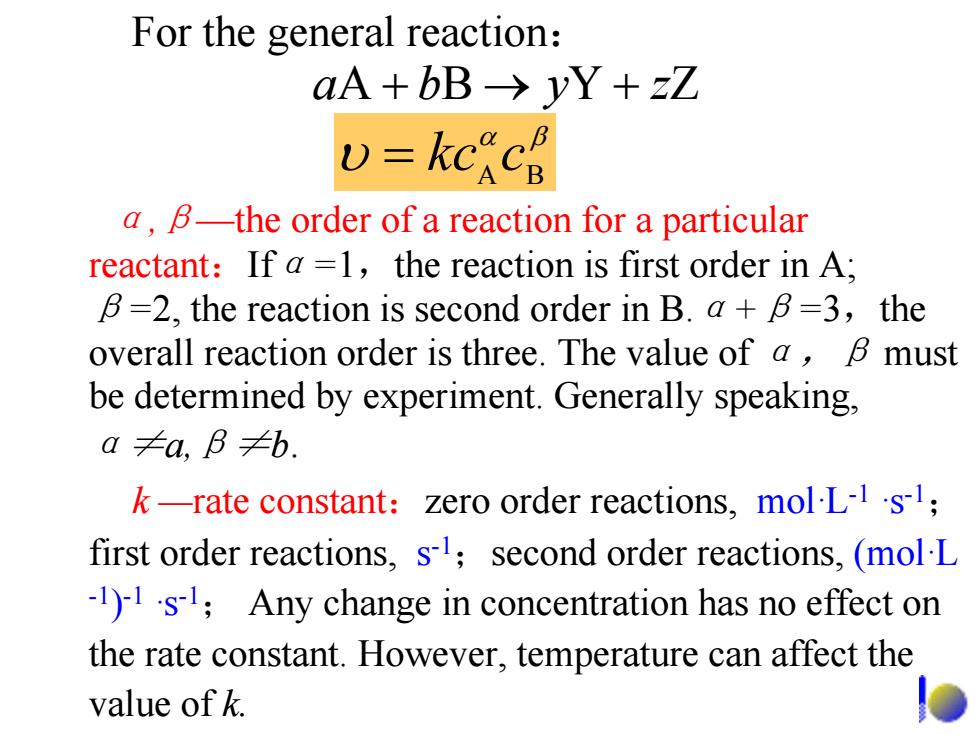
For the general reaction: aA+bB→yY+zZ v=kckc a,B-the order of a reaction for a particular reactant:If a =1,the reaction is first order in A; B=2,the reaction is second order in B.a+B=3,the overall reaction order is three.The value of a,B must be determined by experiment.Generally speaking, a≠a,B≠b. k-rate constant:zero order reactions,mol-L-1's1; first order reactions,s1;second order reactions,(mol L 1)1s;Any change in concentration has no effect on the rate constant.However,temperature can affect the value of k
For the general reaction : α,β—the order of a reaction for a particular reactant:If α = 1 ,the reaction is first order in A; β =2, the reaction is second order in B. α + β = 3 ,the overall reaction order is three. The value of α,β must be determined by experiment. Generally speaking, α≠a,β≠b. k —rate constant:zero order reactions, mol·L-1 ·s-1; first order reactions, s-1;second order reactions, (mol·L -1 )-1 ·s-1; Any change in concentration has no effect on the rate constant. However, temperature can affect the value of k. + ba → y + zZYBA βα υ BA = ckc
3.3 Brief introduction of effect of temperature on reaction rate Arrhenius equation -3.3.1 Arrhenius equation -3.3.2 Application of the Arrhenius equation
3.3.1 Arrhenius equation §3.3 Brief introduction of effect of temperature on reaction rate — Arrhenius equation 3.3.2 Application of the Arrhenius equation

3.3.1 Arrhenius equation aA+bB→yY+zZ The rate expression v=kcacp The factors affecting the reaction rate are as follows:k and c(concentration of reactants) The reaction rate constant k is related with temperature T.Generally,the higher the temperature,the larger the rate constant (exceptions?).However,the rate constant is not linear with temperature
The factors affecting the reaction rate are as follows : k and c (concentration of reactants) The reaction rate constant k is related with temperature T. Generally, the higher the temperature, the larger the rate constant (exceptions?). However, the rate constant is not linear with temperature. The rate expression βα υ BA = ckc 3.3.1 Arrhenius equation + ba y +→ zZYBA