
Preface 1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History,revival and development of inorganic chemistry 1.4 How to study easily and efficiently inorganic chemistry
1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History, revival and development of inorganic chemistry 1.4 How to study easily and efficiently inorganic chemistry Preface

1.1 Chemistry----the central science full of practicability and creativity (1)what is chemistry the study of the properties,compositions and structures of the substances and of the reactions that transform them into other substances (2)Essence and station of chemistry a science full of practicability and creativity Paper making technique(汉朝) ●ancient Chinese Explosive powder(唐朝) technical invention Ceramics(始于汉.唐) Metallurgy(治金,炼丹) Dyeing(染色)
(1) what is chemistry ? the study of the properties, compositions and structures of the substances and of the reactions that transform them into other substances 1.1 Chemistry---- the central science full of practicability and creativity (2) Essence and station of chemistry a science full of practicability and creativity ancient Chinese technical invention Paper making technique ( 汉朝) Explosive powder (唐朝) Ceramics (始于汉. 唐) Metallurgy (冶金, 炼丹) Dyeing (染色)

《本草纲目》 chemistry literatures of ancient Chinese 《天公开物》 Chemistry with modern lives clothing:化学合成纤维;天然纤维;传统的棉、麻、 丝、毛的化学处理;化学颜料 food and drinks:添加剂、甜味剂、防腐剂、色素、香 料、调味剂;维生素和多种微量元素. travel::交通安全 化学是由实用开始,在实用中得到发展,在发展中充 满发明和创造的科学
clothing:化学合成纤维;天然纤维;传统的棉、麻、 丝、毛的化学处理 ;化学颜料 food and drinks:添加剂、甜味剂、防腐剂、色素、香 料、调味剂;维生素和多种微量元素. travel:交通安全 化学是由实用开始,在实用中得到发展,在发展中充 满发明和创造的科学 《本草纲目》 《天公开物》 chemistry literatures of ancient Chinese Chemistry with modern lives
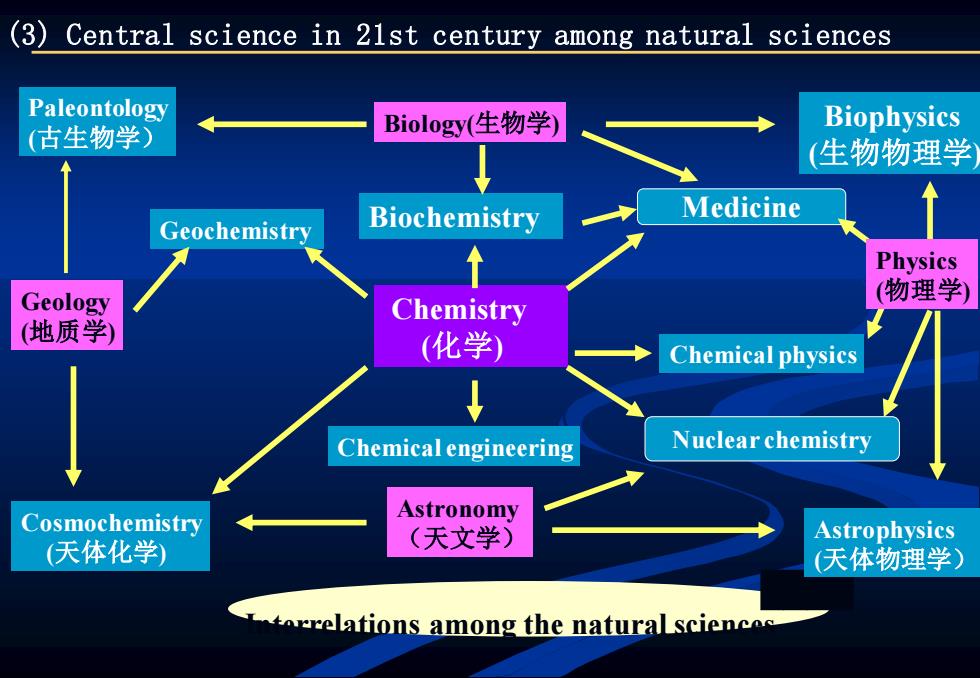
(3)Central science in 21st century among natural sciences Paleontology (古生物学) Biology(生物学) Biophysics (生物物理学 Biochemistry Medicine Geochemistry Physics Geology 物理学) Chemistry (地质学) (化学) Chemical physics Chemical engineering Nuclear chemistry Cosmochemistry Astronomy (天体化学) (天文学) Astrophysics (天体物理学) orrelations among the natural sciencos
Chemistry (化学) Biochemistry Medicine Nuclear chemistry Biophysics (生物物理学) Paleontology (古生物学) Biology(生物学) Chemical physics Chemical engineering Geology (地质学) Geochemistry Cosmochemistry (天体化学) Astronomy (天文学) Physics (物理学) Astrophysics (天体物理学) (3) Central science in 21st century among natural sciences Interrelations among the natural sciences

Preface 1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History,revival and development of inorganic chemistry 1.4 How to study easily but efficiently inorganic chemistry
1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History, revival and development of inorganic chemistry 1.4 How to study easily but efficiently inorganic chemistry Preface
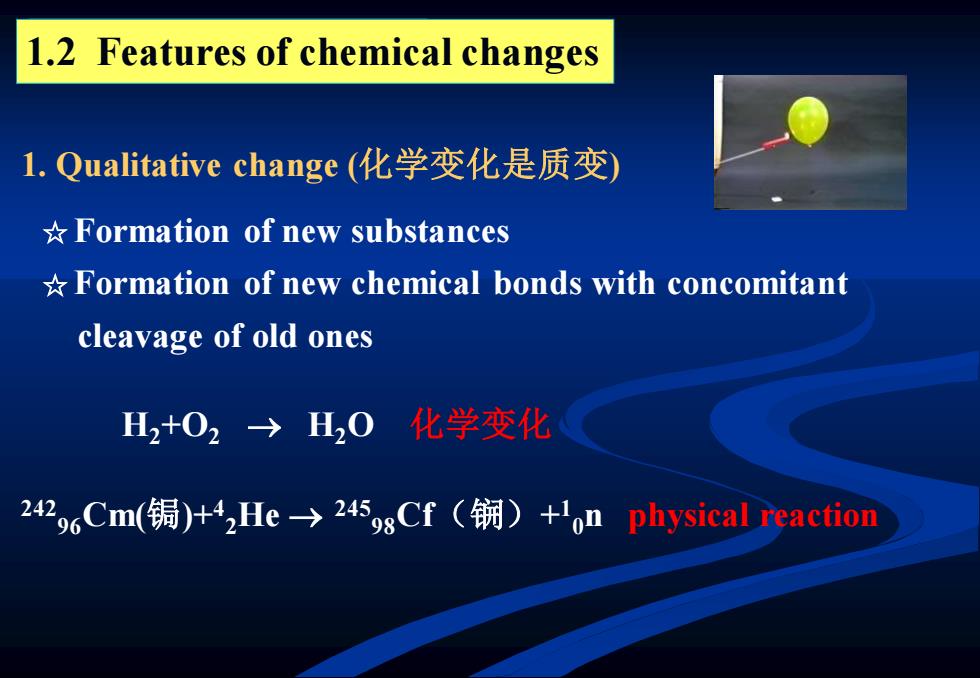
1.2 Features of chemical changes l.Qualitative change(化学变化是质变) *Formation of new substances *Formation of new chemical bonds with concomitant cleavage of old ones H2+02→H20化学变化 242,oCm(锔)+4,He-→245gsCf(锎)+'on physical reaction
1.2 Features of chemical changes 1. Qualitative change (化学变化是质变) 242 96Cm(锔)+4 2He → 245 98Cf(锎)+ 1 0n physical reaction H2+O2 → H2O 化学变化 ☆ Formation of new substances ☆ Formation of new chemical bonds with concomitant cleavage of old ones

2.Chemical reactions obey the law of mass conservation 化学反应过程中各元素的原子数和核外电子的总数没有变化 ,并且参与反应的各种物质之间有确定的计量关系 3.Chemical reactions always occur companying energy changes 化学键的断裂和形成,电子的转移伴随能量的吸收和释放,如 石油燃烧等
2. Chemical reactions obey the law of mass conservation 化学反应过程中各元素的原子数和核外电子的总数没有变化 ,并且参与反应的各种物质之间有确定的计量关系 3. Chemical reactions always occur companying energy changes 化学键的断裂和形成,电子的转移伴随能量的吸收和释放, 如 石油燃烧等

Preface 1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History,revival and development of inorganic chemistry 1.4 How to study easily but efficiently inorganic chemistry
1.1 Chemistry---the central science full of practicability and creativity 1.2 Features of chemical changes 1.3 History, revival and development of inorganic chemistry 1.4 How to study easily but efficiently inorganic chemistry Preface
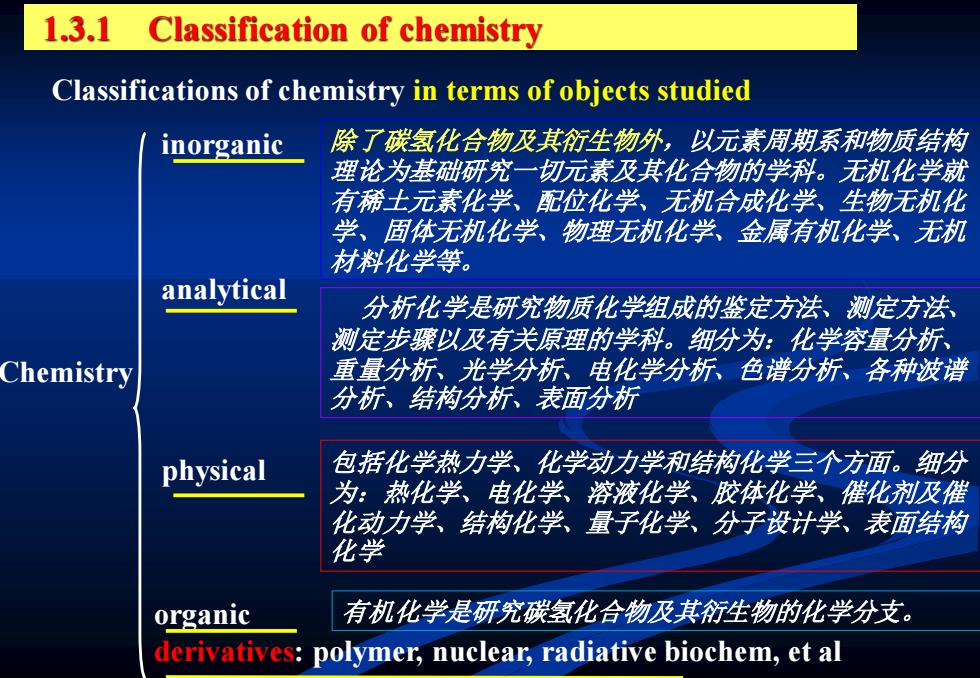
1.3.1 Classification of chemistry Classifications of chemistry in terms of objects studied inorganic 除了碳氢化合物及其衍生物外,以元素周期系和物质结构 理论为基础研究一切元素及其化合物的学科。无机化学就 有稀士元素化学、配位化学、无机合成化学、生物无机化 学、固体无机化学、物理无机化学、金属有机化学、无机 材料化学等。 analytical 分析化学是研究物质化学组成的鉴定方法、测定方法 测定步骤以及有关原理的学科。细分为:化学容量分析、 Chemistry 重量分析、光学分析、电化学分析、色谱分析、各种波谱 分析、结构分析、表面分析 physical 包括化学热力学、化学动力学和结构化学三个方面。细分 为:热化学、电化学、溶液化学、胶体化学、催化剂及催 化动力学、结构化学、量子化学、分子设计学、表面结构 化学 organic 有机化学是研究碳氢化合物及其衍生物的化学分支。 derivatives:polymer,nuclear,radiative biochem,et al
inorganic analytical physical organic derivatives: polymer, nuclear, radiative biochem, et al Chemistry 除了碳氢化合物及其衍生物外,以元素周期系和物质结构 理论为基础研究一切元素及其化合物的学科。无机化学就 有稀土元素化学、配位化学、无机合成化学、生物无机化 学、固体无机化学、物理无机化学、金属有机化学、无机 材料化学等。 分析化学是研究物质化学组成的鉴定方法、测定方法、 测定步骤以及有关原理的学科。细分为:化学容量分析、 重量分析、光学分析、电化学分析、色谱分析、各种波谱 分析、结构分析、表面分析 包括化学热力学、化学动力学和结构化学三个方面。细分 为:热化学、电化学、溶液化学、胶体化学、催化剂及催 化动力学、结构化学、量子化学、分子设计学、表面结构 化学 有机化学是研究碳氢化合物及其衍生物的化学分支。 Classifications of chemistry in terms of objects studied 1.3.1 Classification of chemistry
0 2 IIA IIIA IVA VA VIA VIIA He 5 6 7 8 g 10 Be B C N F Ne 11 12 13 14 15 16 17 18 3 Na Mg IlIB IVB VB VIB VIIB VIIB 1B IIB Si P S CI Ar 19 20 21 22 23 24 25 26 27 28 29 30 3T■ 32 33 34 35 36 K Ca Sc i Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 39 40 41 2 43 44 45 46 47 48 49 50 51 2 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Xe 56 57 72 73 74 75 76 77 78 79 80 81 83 84 85 86 6 Cs Ba *La Hf Ta W Re Os Ir Pt Au Hg T Pb Bi At Rn 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 Ra +Ac 104 105 106 107 108 109 110 111 112 114 116 118 "Lanthanide 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Series Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu +Actinide 91 92 93 94 95 96 97 98 gg 100 101T 102 103 Series Th Pa Np Pu Am Cm Bk C Es Fm Md No Lr