
Chap 11 Coordination Compound Structures 11.1 Configurations and Magnetism of Coordination Compounds X 11.2 Chemical Bonding Theory in Complexes
Chap 11 Coordination Compound Structures §11.2 Chemical Bonding Theory in Complexes §11.1 Configurations and Magnetism of Coordination Compounds
11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1.configurations 2.isomers 11.1.2 Magnetism of Coordination Compounds
11.1 Configurations and Magnetism of Coordination Compounds 11.1.1 Configurations of Coordination Compounds 1. configurations 2. isomers 11.1.2 Magnetism of Coordination Compounds
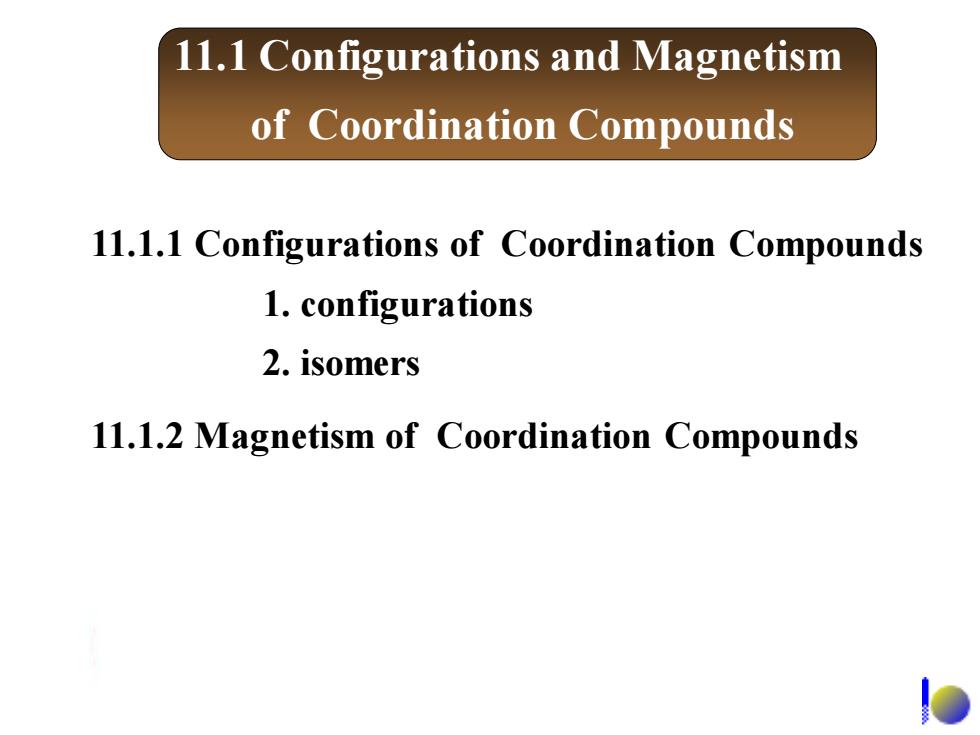
11.1.1 Configurations of coordination compounds 1.Geometric arrangements of atoms in a coordination compound: 与配位数及配体种类有关 0◆0 00-0 88 dodecahedral 正十二面体形 linear tetrahedral Square planer octahedral (8个顶点) NH3 12 2 Ag(NH3)2 -NH3 H
11.1.1 Configurations of coordination compounds 1. Geometric arrangements of atoms in a coordination compound: 与配位数及配体种类有关 linear Square planer tetrahedral octahedral dodecahedral 正十二面体形 (8个顶点) + 3 2 Ag(NH )
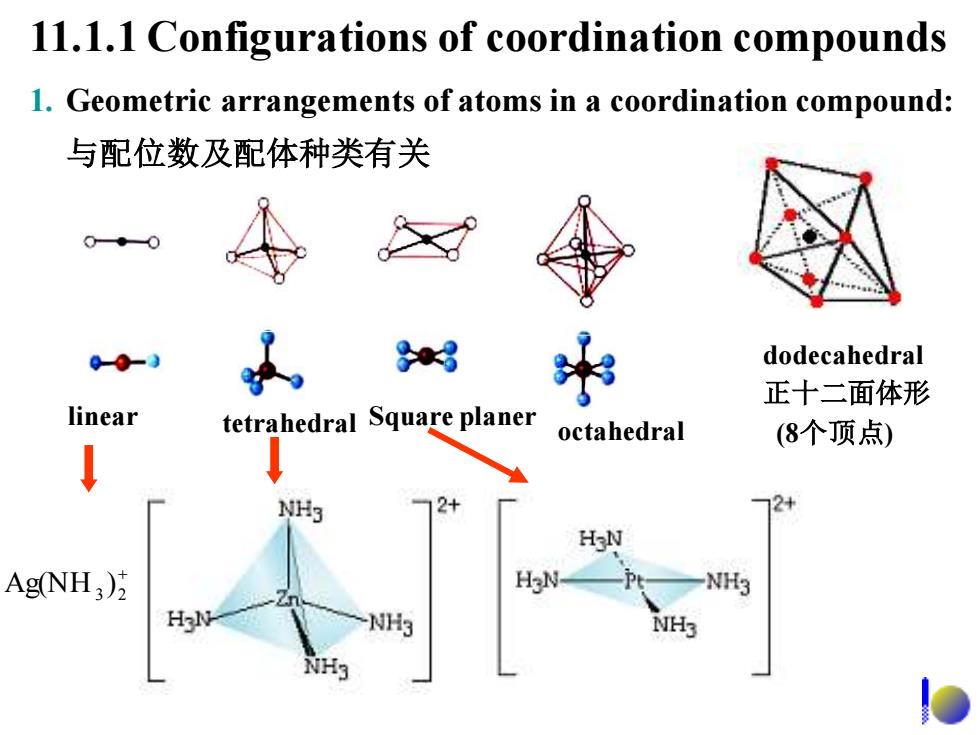
H CH H 八面体形 CoEDTA Fe(CN) O HO-C-H2C CH2-C-OH HO-C-H2C N-CH.CHN CH2-C-OH 0 D
O HO-C-H2C O HO-C-H2C N-CH2 -CH2 -N O CH2 -C-OH O CH2 -C-OH 乙二胺四乙酸 八面体形 3− Fe(CN)6
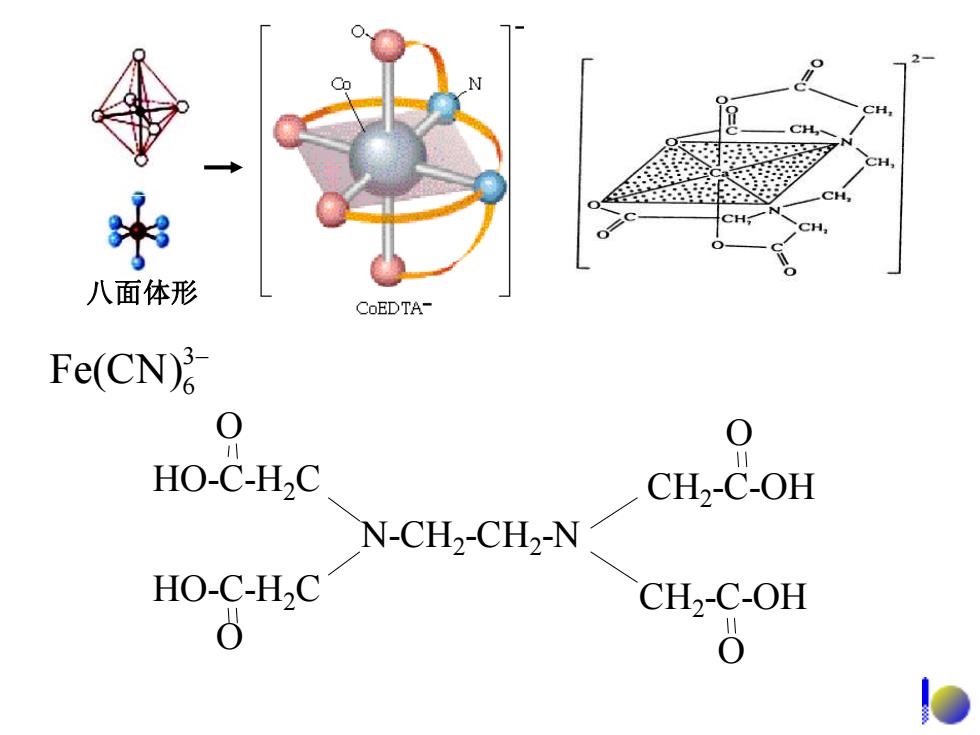
Hgl; N SbCI Square pyramidal Triangular 四方锥形 平面三角形 Triangle bipyramidal 三角双锥形 Fe(CO)s
Triangular 平面三角形 Square pyramidal 四方锥形 Triangle bipyramidal 三角双锥形 − HgI 3 2− SbCl5 Fe(CO)5
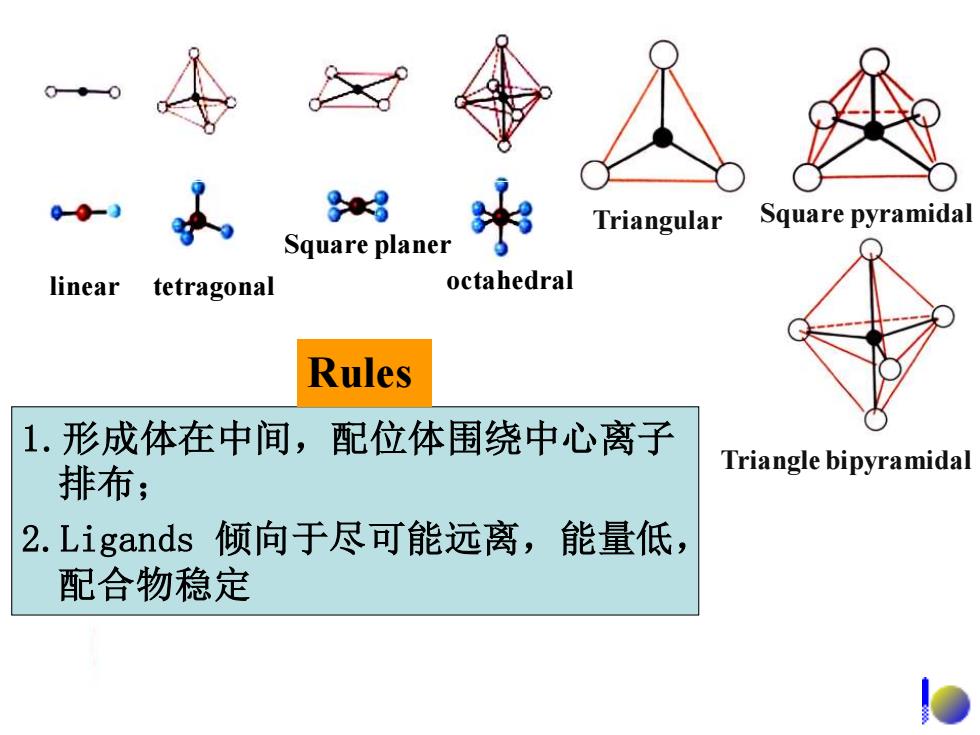
o+o 83 Triangular Square pyramidal Square planer linear tetragonal octahedral Rules 1.形成体在中间,配位体围绕中心离子 Triangle bipyramidal 排布; 2.Ligands倾向于尽可能远离,能量低, 配合物稳定
linear Square planer tetragonal octahedral Triangular Square pyramidal Triangle bipyramidal 1.形成体在中间,配位体围绕中心离子 排布; 2.Ligands 倾向于尽可能远离,能量低, 配合物稳定 Rules
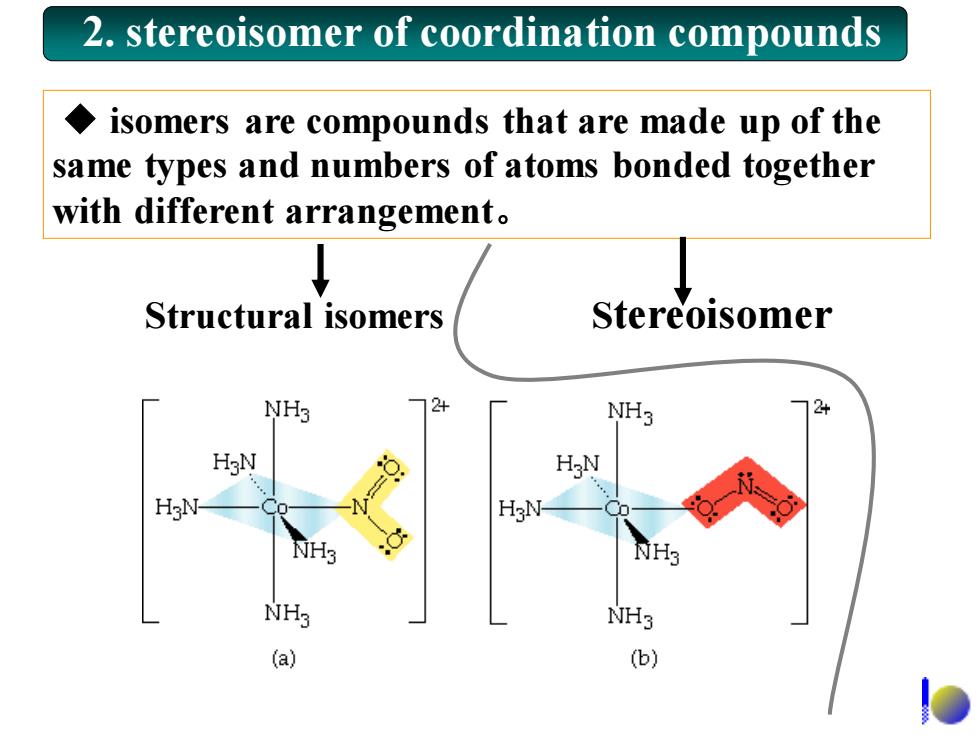
2.stereoisomer of coordination compounds isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement. Structural isomers Stereoisomer NH3 24 NH3 HgN H N NH3 NH3 NH3 (a) (b)
◆ isomers are compounds that are made up of the same types and numbers of atoms bonded together with different arrangement。 2. stereoisomer of coordination compounds Structural isomers Stereoisomer
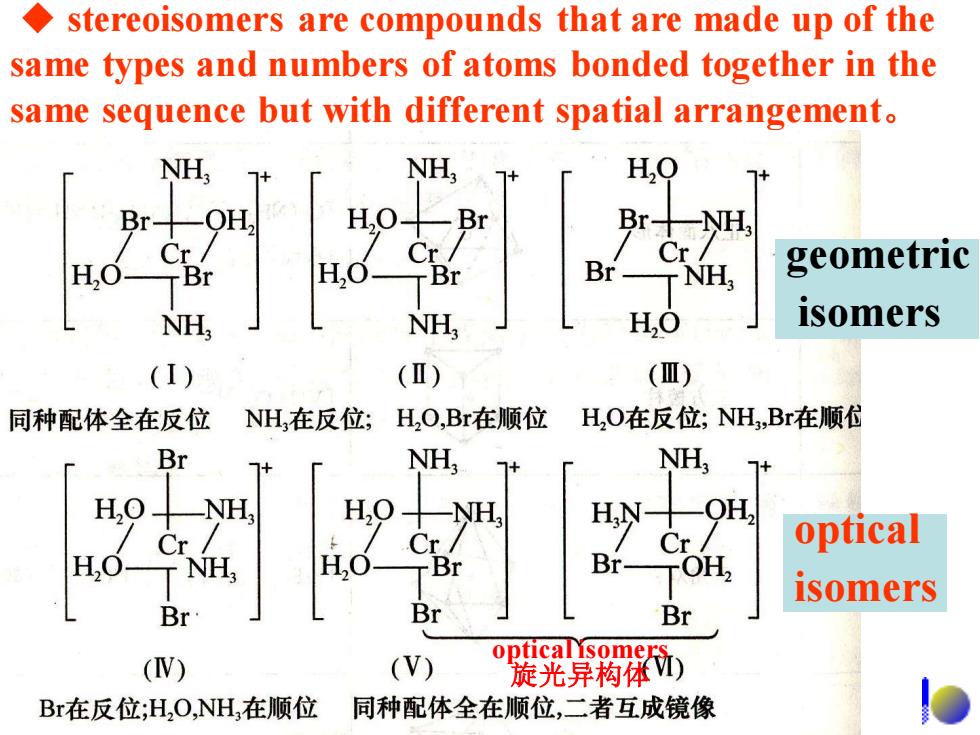
stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement. NH, NH, H,0 Br-OH, H.O Br Br H Cr/ Cr/ Cr H,0 H,0 Br TNH geometric NH NH HO isomers (I) (I) (Ⅲ) 同种配体全在反位 NH在反位; H,O,Br在顺位 H,O在反位;NH,Br在顺 Br NH, NH, 7+ NH, H,9—WH HN OH, Cr Cr/ optical H,C T NH HO- Cr/ TBr Br- TOH isomers Br Br Br (V) (V) 乳异粉 Br在反位;H2O,NH在顺位 同种配体全在顺位,二者互成镜像
◆ stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangement。 optical isomers 旋光异构体 geometric isomers optical isomers
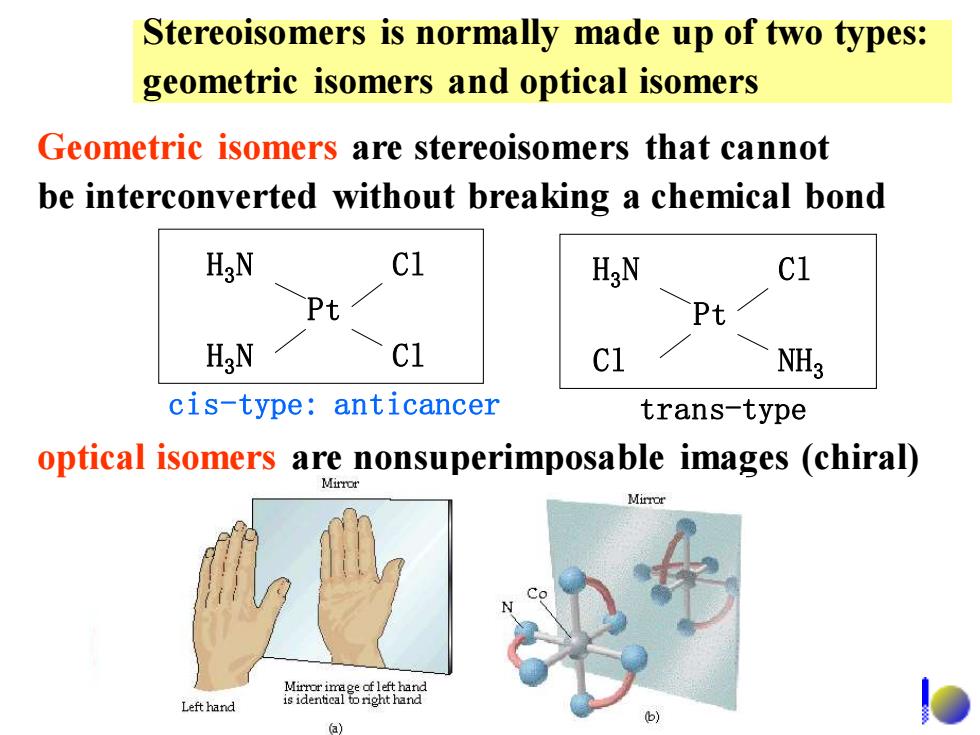
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond HgN Cl HgN C1 Pt Pt HgN c1 NH3 cis-type:anticancer trans-type optical isomers are nonsuperimposable images (chiral) Mirror Left hand a)
Stereoisomers is normally made up of two types: geometric isomers and optical isomers Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond optical isomers are nonsuperimposable images (chiral) H3N H3N Pt Cl Cl H3N Cl Pt Cl NH3 cis-type: anticancer trans-type

思考: ①配位数为4的正四面体结构的配位 化合物是否有顺、反异构体? no ②配位数为6的八面体结构的配位化 合物是否有顺、反异构体? yes
思考: ① 配位数为4的正四面体结构的配位 化合物是否有顺、反异构体? no ② 配位数为6的八面体结构的配位化 合物是否有顺、反异构体? yes