
Chapter 12 The alkali and alkaline earth meta X 12.1 The elementary substances 12.2 The properties of the alkali and alkaline earth compounds X$12.3 The particularity of Li and Be The diagonal relationship
§12.3 The particularity of Li and Be The diagonal relationship §12.2 The properties of the alkali and alkaline earth compounds §12.1 The elementary substances Chapter 12 The alkali and alkaline earth metal

12.1 The elementary substances 12.1.1 Properties of the elementary substances 1.Physical properties ☆Metallic luster; ☆Low densities;. ☆Soft; ☆Low melting point;: *Good conductor of heat and electricity Na Li K
§12.1.1 Properties of the elementary substances Na Li K 1.Physical properties §12.1 The elementary substances ☆Metallic luster; ☆Low densities; ☆Soft; ☆Low melting point; ☆Good conductor of heat and electricity

Rb 181 Low melting point Be Mg Ca 98 6 29 Sr Ba Li Na K Rb Cs Fr
Be Mg Ca Sr Ba Rb Cs Low melting point Li Na K Rb Cs Fr

2.Chemical properties: React with oxygen,sulfur,nitrogen and halogen to form their corresponding compounds. The elementary substances form the corresponding oxides when they burn in air: Li2O Na202 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 Na,O, Pale yellow Pale yellow Magnesium burning in air
The elementary substances form the corresponding oxides when they burn in air: Li2O Na2O2 KO2 RbO2 CsO2 BeO MgO CaO SrO BaO2 • React with oxygen, sulfur, nitrogen and halogen to form their corresponding compounds. 2.Chemical properties: Magnesium burning in air Li2O Na2O2 KO2 Pale yellow Pale yellow

.React with water 2M+2H,O->2MOH+H2(g) Li Na K Bromothymol blue indicator 溴百里酚兰指示剂 Ca
•React with water Li Na K Ca 2M + 2H2O → 2MOH + H2 (g) Bromothymol blue indicator 溴百里酚兰 指示剂
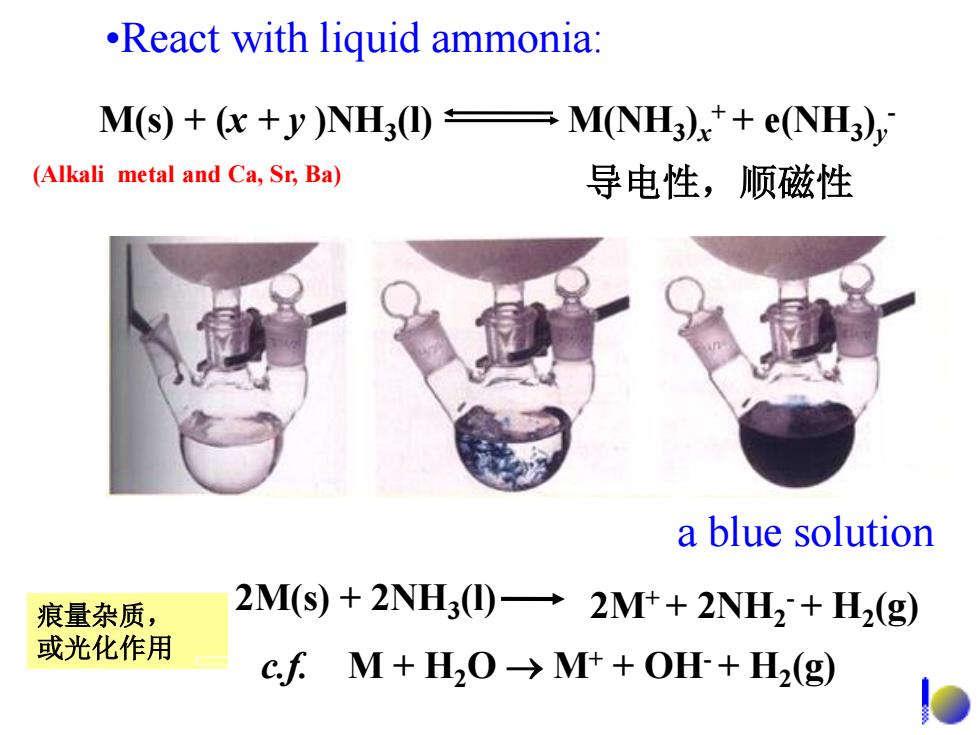
React with liquid ammonia: M(S+(c+y)NH3①= M(NH3)x+e(NH3) (Alkali metal and Ca,Sr,Ba) 导电性,顺磁性 a blue solution 痕量杂质, 2M(s)+2NH3(I)2M++2NH2+H2(g) 或光化作用 cfM+H20→Mt+OH+H2(g)
•React with liquid ammonia: a blue solution 2M(s) + 2NH3 (l) 2M+ + 2NH2 - + H2 (g) c.f. M + H2O M+ + OH- + H2 (g) M(s) + (x + y )NH3 (l) M(NH3 )x + + e(NH3 )y - 导电性,顺磁性 痕量杂质, 或光化作用 (Alkali metal and Ca, Sr, Ba)
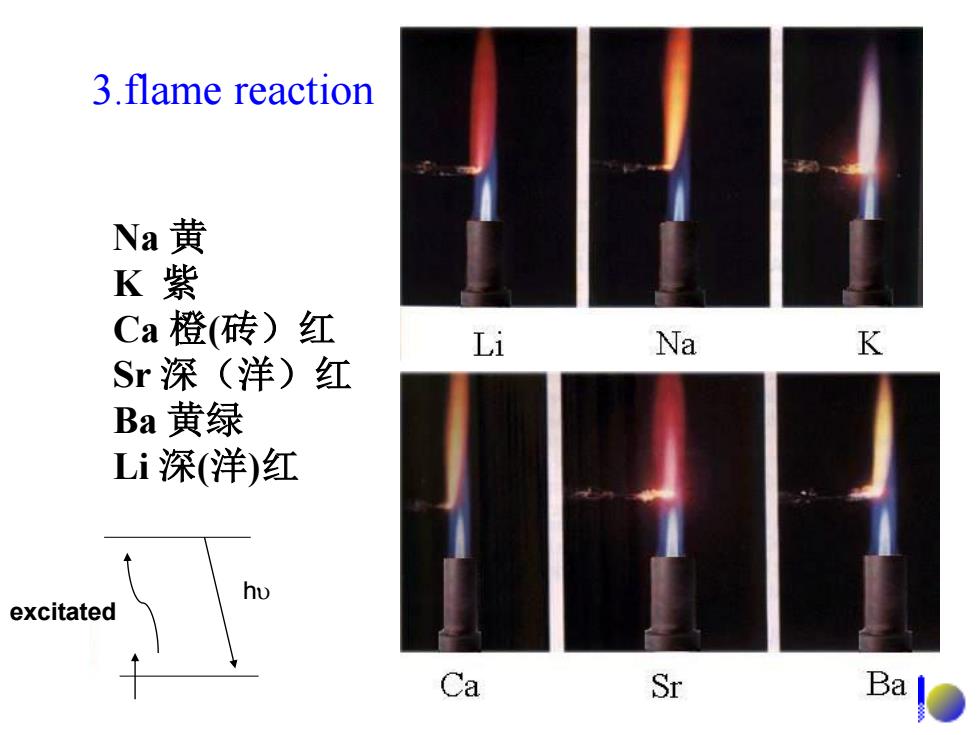
3.flame reaction Na黄 K紫 Ca橙(砖)红 Li Na K Sr深(洋)红 Ba黄绿 Li深(洋)红 excitated Ca Sr Ba
3.flame reaction Na 黄 K 紫 Ca 橙(砖)红 Sr 深(洋)红 Ba 黄绿 Li 深(洋)红 excitated h

S 12.1.2 The existence and preparation of s-block elements The s-block elements exist in minerals: Albite(钠长石):Na[AISi,Og] Potash feldspar(钾长石):K[ASi,Og] Carnallite(光卤石):KCl.MgCl2·6H2O Alunite(明矾石):K(A1O)3(SO4)2·3H2O Spodumene(锂恽石):LiAl(SiO3)2
The s-block elements exist in minerals: Na AlSi 3 O8 K AlSi 3 O8 KClMgCl 2 6H2 O K(AlO) 3 (SO4 )2 3H2 O 3 2 Spodumene (锂辉石): LiAl(SiO ) Albite (钠长石): Potash feldspar (钾长石): Carnallite (光卤石): Alunite (明矾石): § 12.1.2 The existence and preparation of s-block elements

Beyl(绿柱石):Be3A12(SiO3)6 Magnesite(菱镁矿):MgCO; Gess0(石膏):CaSO4·2H2O Marble(大理石):CaCO Fluorite(萤石):CaF2 Celestite(天青石):SrSO4 Barite(重晶石):BaSO4
Beryl (绿柱石): Magnesite (菱镁矿): Fluorite (萤石): Celestite (天青石): Marble (大理石): 3 2 3 6 Be Al (SiO ) MgCO3 CaSO4 2H2 O CaCO3 CaF2 SrSO4 BaSO4 Gesso (石膏): Barite (重晶石):

Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. In addition,热还原法,金属置换法,热分解法etal (See page 465-466
Preparation: The alkali and alkaline earth elemental substances are most conveniently obtained from molten salts by electrolysis. (See page 465-466 ) In addition, 热还原法, 金属置换法, 热分解法 et al