Chapter 9 Molecular Structure ק9.1 Lewis structure Theory ק9.2 Valence Bond Theory ק9.3 Valence Shell Electron Pair Repulsion (VSEPR)Theory x§9.4 Molecular Orbital Theory ק9.5 Bond Parameters
Chapter 9 Molecular Structure §9.2 Valence Bond Theory §9.5 Bond Parameters §9.4 Molecular Orbital Theory §9.3 Valence Shell Electron Pair Repulsion (VSEPR) Theory §9.1 Lewis structure Theory
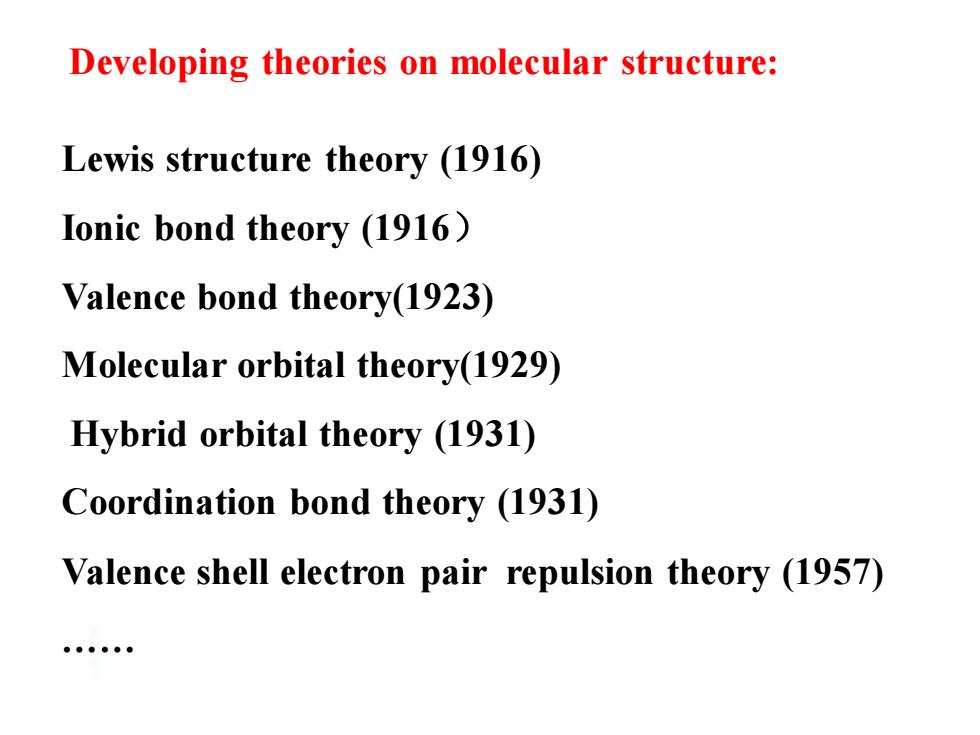
Developing theories on molecular structure: Lewis structure theory (1916) Ionic bond theory (1916) Valence bond theory(1923) Molecular orbital theory(1929) Hybrid orbital theory (1931) Coordination bond theory (1931) Valence shell electron pair repulsion theory (1957)
Developing theories on molecular structure: Lewis structure theory (1916) Ionic bond theory (1916) Valence bond theory(1923) Molecular orbital theory(1929) Hybrid orbital theory (1931) Coordination bond theory (1931) Valence shell electron pair repulsion theory (1957) ……
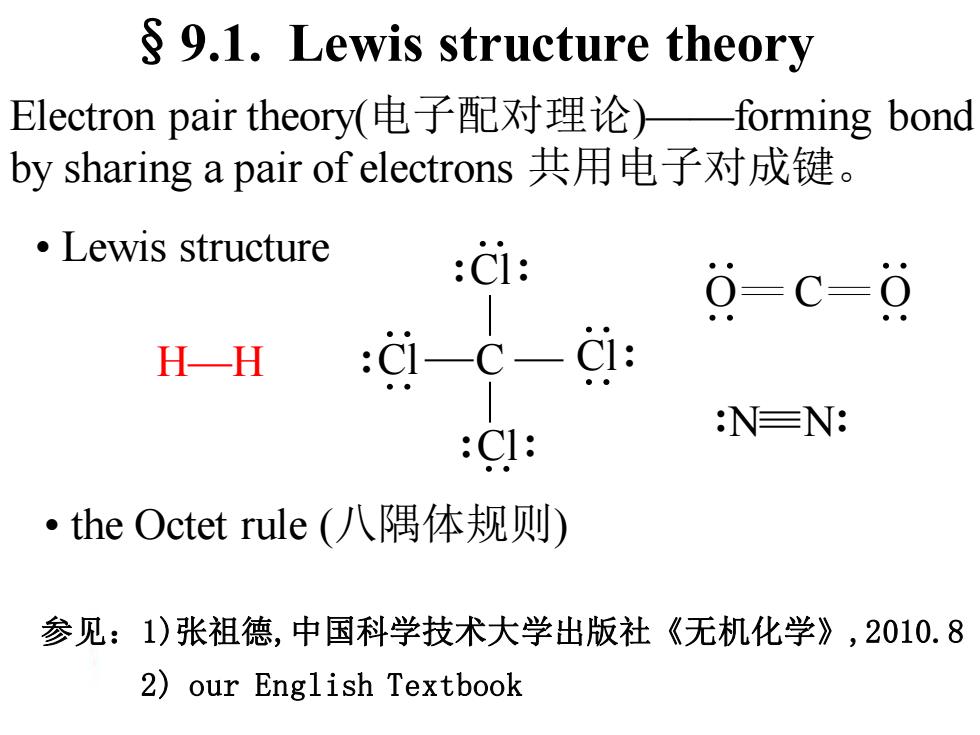
9.1.Lewis structure theory Electron pair theory(电子配对理论)一forming bond by sharing a pair of electrons共用电子对成键。 ·Lewis structure :C1: 0=c-9 H-H :i一C-i: NEN: :CI: ·he Octet rule(八隅体规则) 参见:1)张祖德,中国科学技术大学出版社《无机化学》,2010.8 2)our English Textbook
§9.1. Lewis structure theory Electron pair theory(电子配对理论)——forming bond by sharing a pair of electrons 共用电子对成键。 • Lewis structure • the Octet rule (八隅体规则) H—H :Cl Cl: :Cl: :Cl: C :N N: O C O 参见:1)张祖德,中国科学技术大学出版社《无机化学》,2010.8 2) our English Textbook
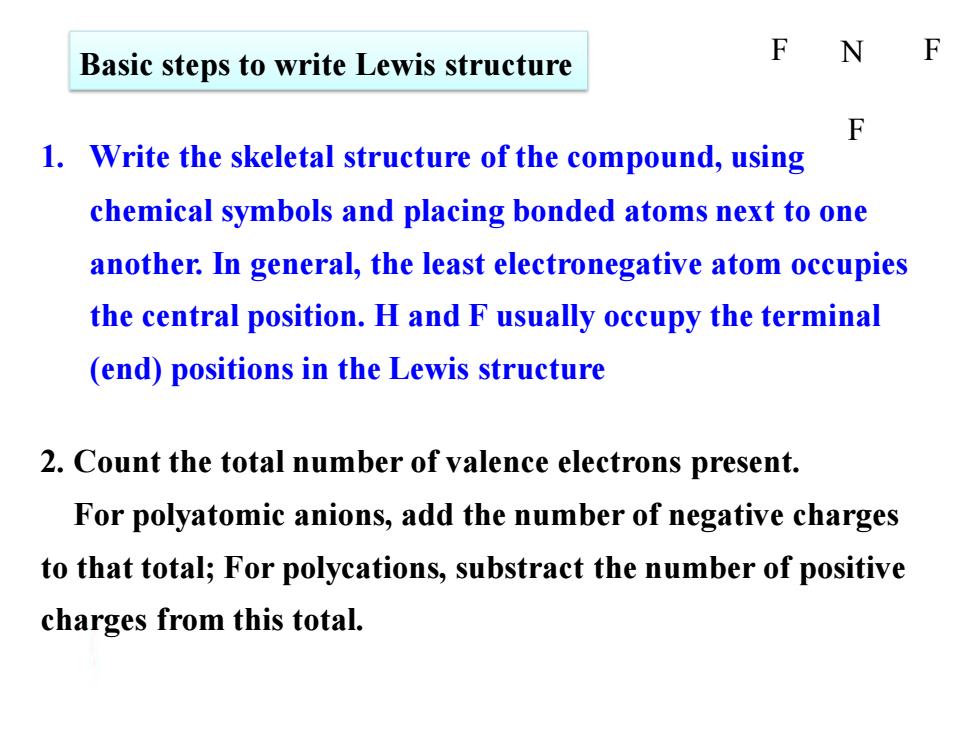
F Basic steps to write Lewis structure N F 1.Write the skeletal structure of the compound,using chemical symbols and placing bonded atoms next to one another.In general,the least electronegative atom occupies the central position.H and F usually occupy the terminal (end)positions in the Lewis structure 2.Count the total number of valence electrons present. For polyatomic anions,add the number of negative charges to that total;For polycations,substract the number of positive charges from this total
Basic steps to write Lewis structure 1. Write the skeletal structure of the compound, using chemical symbols and placing bonded atoms next to one another. In general, the least electronegative atom occupies the central position. H and F usually occupy the terminal (end) positions in the Lewis structure 2. Count the total number of valence electrons present. For polyatomic anions, add the number of negative charges to that total; For polycations, substract the number of positive charges from this total. F N F F
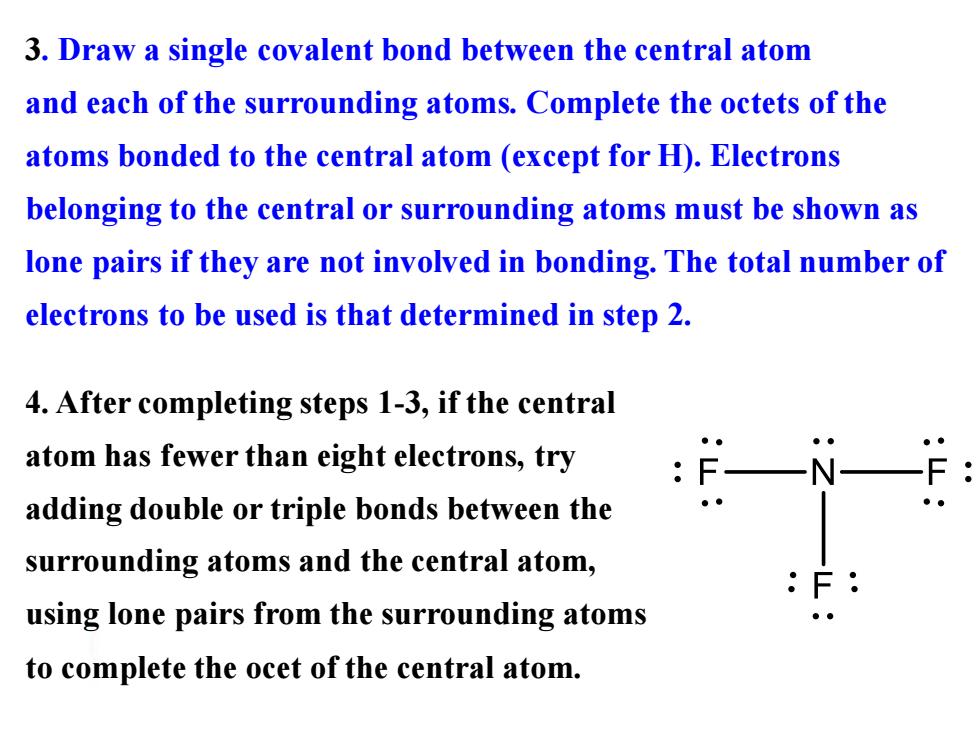
3.Draw a single covalent bond between the central atom and each of the surrounding atoms.Complete the octets of the atoms bonded to the central atom (except for H).Electrons belonging to the central or surrounding atoms must be shown as lone pairs if they are not involved in bonding.The total number of electrons to be used is that determined in step 2. 4.After completing steps 1-3,if the central atom has fewer than eight electrons,try adding double or triple bonds between the surrounding atoms and the central atom, using lone pairs from the surrounding atoms to complete the ocet of the central atom
3. Draw a single covalent bond between the central atom and each of the surrounding atoms. Complete the octets of the atoms bonded to the central atom (except for H). Electrons belonging to the central or surrounding atoms must be shown as lone pairs if they are not involved in bonding. The total number of electrons to be used is that determined in step 2. 4. After completing steps 1-3, if the central atom has fewer than eight electrons, try adding double or triple bonds between the surrounding atoms and the central atom, using lone pairs from the surrounding atoms to complete the ocet of the central atom
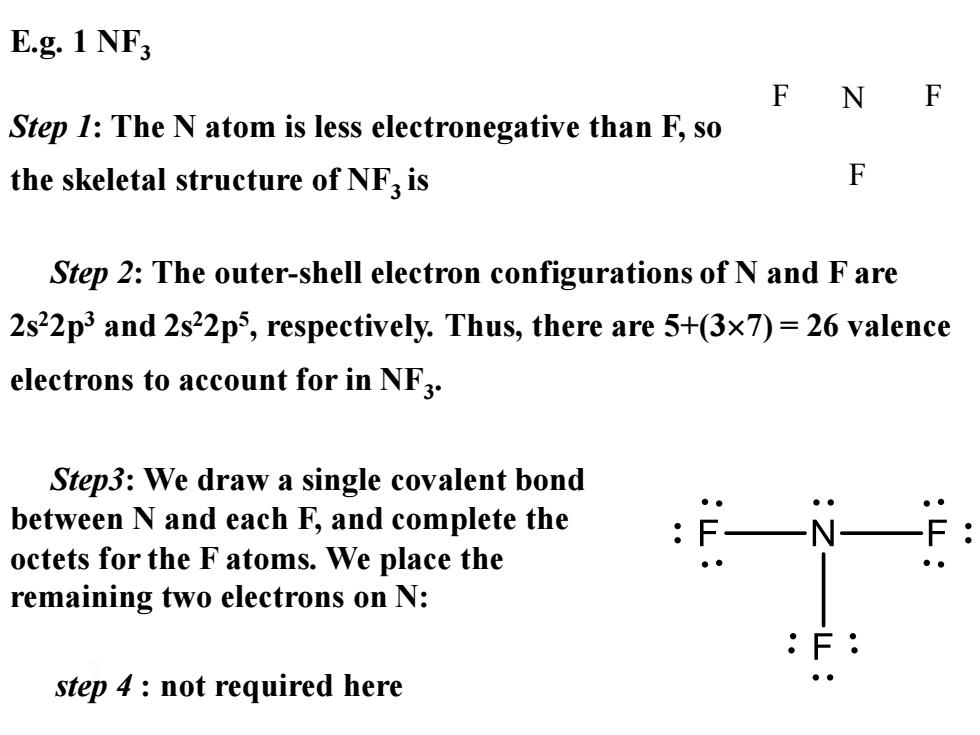
E.g.1 NF F F Step 1:The N atom is less electronegative than F,so the skeletal structure of NF3 is F Step 2:The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5,respectively.Thus,there are 5+(3x7)=26 valence electrons to account for in NF3. Step3:We draw a single covalent bond between N and each F,and complete the octets for the F atoms.We place the remaining two electrons on N: step 4 not required here ●
E.g. 1 NF3 Step 1: The N atom is less electronegative than F, so the skeletal structure of NF3 is F N F F Step3: We draw a single covalent bond between N and each F, and complete the octets for the F atoms. We place the remaining two electrons on N: Step 2: The outer-shell electron configurations of N and F are 2s22p3 and 2s22p5 , respectively. Thus, there are 5+(37) = 26 valence electrons to account for in NF3 . step 4 : not required here
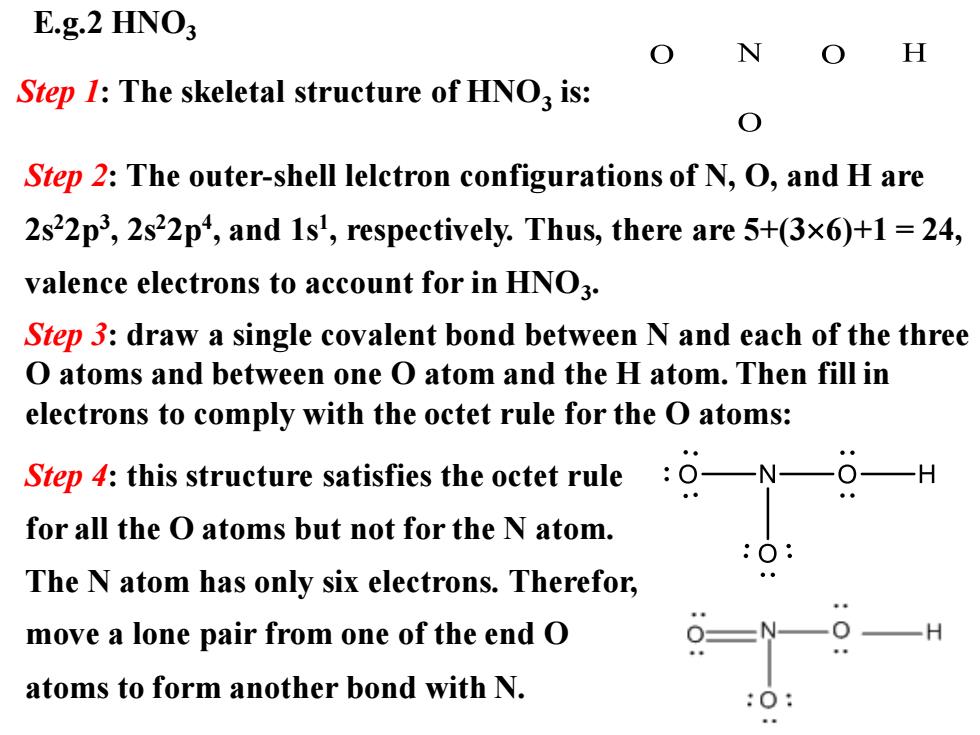
E.g.2 HNO3 N H Step 1:The skeletal structure of HNO3 is: Step 2:The outer-shell lelctron configurations of N,O,and H are 2s22p3,2s22p4,and 1s',respectively.Thus,there are 5+(3x6)+1=24, valence electrons to account for in HNO3. Step 3:draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom.Then fill in electrons to comply with the octet rule for the O atoms: Step 4:this structure satisfies the octet rule O-N- for all the O atoms but not for the N atom. The N atom has only six electrons.Therefor, move a lone pair from one of the end O atoms to form another bond with N
E.g.2 HNO3 Step 1: The skeletal structure of HNO3 is: Step 2: The outer-shell lelctron configurations of N, O, and H are 2s22p3 , 2s22p4 , and 1s1 , respectively. Thus, there are 5+(36)+1 = 24, valence electrons to account for in HNO3 . Step 4: this structure satisfies the octet rule for all the O atoms but not for the N atom. The N atom has only six electrons. Therefor, move a lone pair from one of the end O atoms to form another bond with N. Step 3: draw a single covalent bond between N and each of the three O atoms and between one O atom and the H atom. Then fill in electrons to comply with the octet rule for the O atoms:
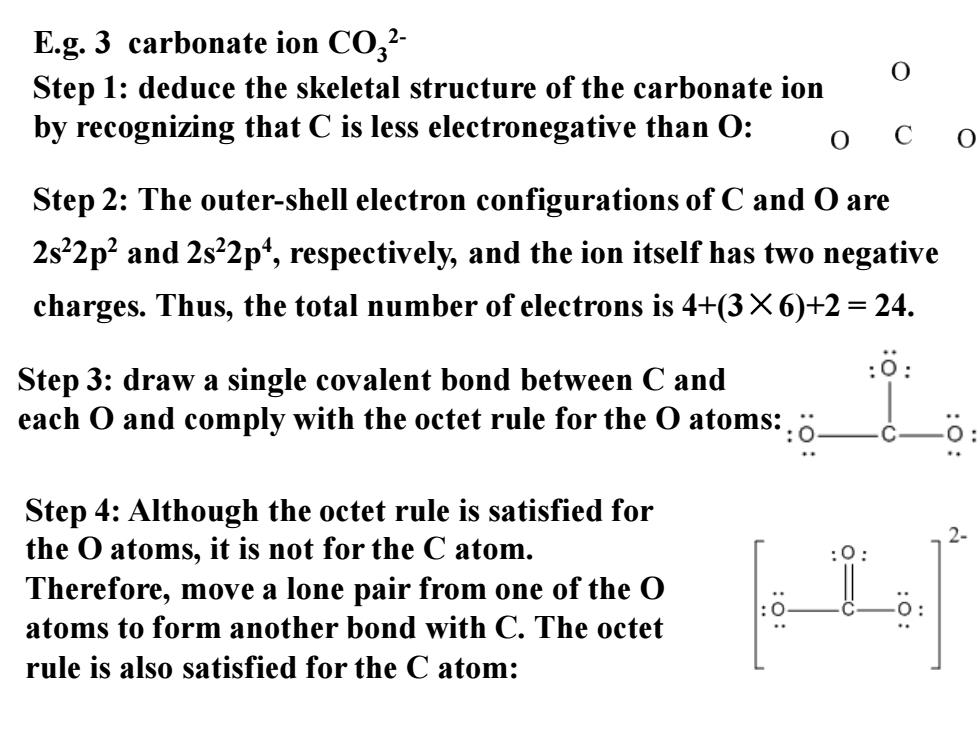
E.g.3 carbonate ion CO2- Step 1:deduce the skeletal structure of the carbonate ion by recognizing that C is less electronegative than O: Step 2:The outer-shell electron configurations of C and O are 2s22p2 and 2s22p4,respectively,and the ion itself has two negative charges.Thus,the total number of electrons is 4+(3X6)+2 24. Step 3:draw a single covalent bond between C and each O and comply with the octet rule for the O atoms::. Step 4:Although the octet rule is satisfied for the O atoms,it is not for the C atom. Therefore,move a lone pair from one of the O atoms to form another bond with C.The octet rule is also satisfied for the C atom:
Step 2: The outer-shell electron configurations of C and O are 2s22p2 and 2s22p4 , respectively, and the ion itself has two negative charges. Thus, the total number of electrons is 4+(3×6)+2 = 24. Step 4: Although the octet rule is satisfied for the O atoms, it is not for the C atom. Therefore, move a lone pair from one of the O atoms to form another bond with C. The octet rule is also satisfied for the C atom: E.g. 3 carbonate ion CO3 2- Step 1: deduce the skeletal structure of the carbonate ion by recognizing that C is less electronegative than O: Step 3: draw a single covalent bond between C and each O and comply with the octet rule for the O atoms:
Exceptions to the octet rule:BF3,PCls,SF et al B:2S22P1,F:2S22P5 The octet rule and Lewis structure do not present a complete picture of covalent bonding
Exceptions to the octet rule: BF3 , PCl5 , SF6 et al B: 2S22P1 ; F: 2S22P5 The octet rule and Lewis structure do not present a complete picture of covalent bonding

§9.2 Valence Bond Theory (VB) -9.2.1 The Essence and Characteristics of Covalent Bond 9.2.2 The Types of Covalent Bond 9.2.3 Hybrid Orbits
9.2.1 The Essence and Characteristics of Covalent Bond §9.2 Valence Bond Theory (VB) 9.2.3 Hybrid Orbits 9.2.2 The Types of Covalent Bond