Chapter 14 The p-block elements(I) X 14.1 The elements of nitrogen family x 14.2 The elements of oxygen family
Chapter 14 The p-block elements(Ⅱ) §14.2 The elements of oxygen family §14.1 The elements of nitrogen family

14.1 The elements of nitrogen family 14.1.1 Outline on nitrogen family 14.1.2 The elemental substances of nitrogen family 14.1.3 The compounds of nitrogen 14.1.4 The compounds of phosphorus -14.1.5 The compounds ofAs,Sb and Bi
14.1.5 The compounds of As, Sb and Bi 14.1.4 The compounds of phosphorus 14.1.3 The compounds of nitrogen 14.1.2 The elemental substances of nitrogen family 14.1.1 Outline on nitrogen family §14.1 The elements of nitrogen family
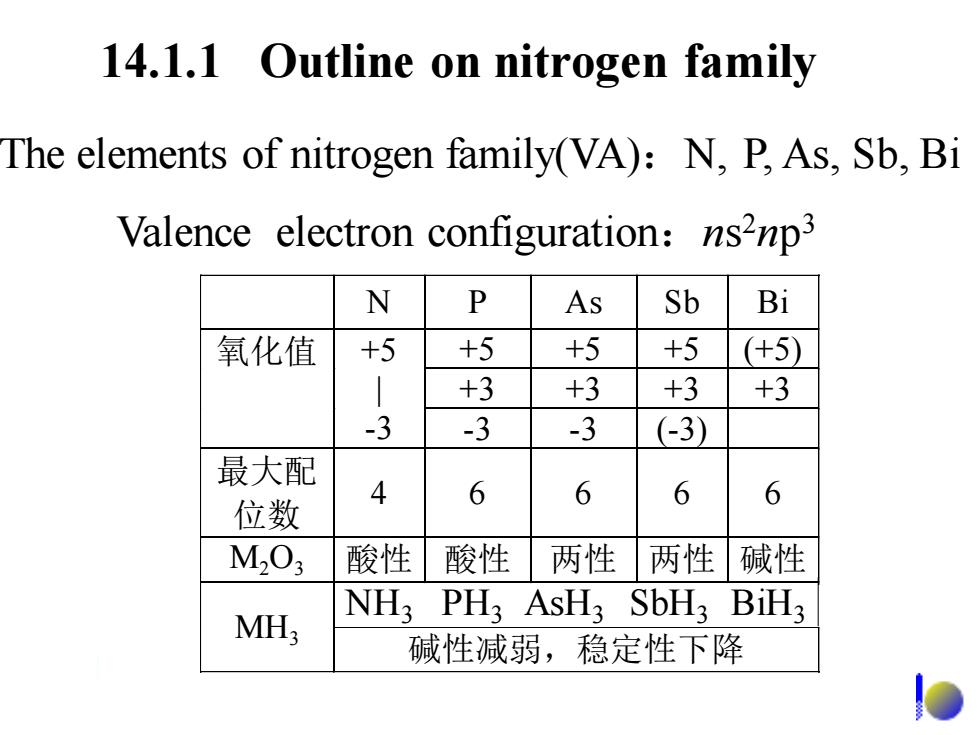
14.1.1 Outline on nitrogen family The elements of nitrogen family(VA):N,P,As,Sb,Bi Valence electron configuration:ns2np3 N P As Sb Bi 氧化值 +5 +5 +5 +5 (+5) +3 +3 +3 +3 -3 -3 -3 (-3) 最大配 位数 4 6 6 6 6 M03 酸性 酸性 两性 两性 碱性 MH; NH3 PH3 AsH3 SbH3 BiH3 碱性减弱,稳定性下降
14.1.1 Outline on nitrogen family The elements of nitrogen family(VA):N, P, As, Sb, Bi Valence electron configuration:ns 2np 3 N P As Sb Bi +5 +5 +5 (+5) +3 +3 +3 +3 氧化值 +5 | -3 -3 -3 (-3) 最大配 位数 4 6 6 6 6 M2O3 酸性 酸性 两性 两性 碱性 氨 膦 胂 SbH3 BiH3 MH3 碱性减弱,稳定性下降 NH3 PH3 AsH3 SbH3 BiH3

14.1.2 The elemental substances of nitrogen family N2 is colorless and odorless gas.N2 is chemically inert.It will react directly with many metals (such as Li,Ca,Mg)when heated to give ionic nitrides.Nitrogen is also easily converted to a liquid (b.p,-195.8'C)and can dissolve in water slightly
N2 is colorless and odorless gas. N2 is chemically inert. It will react directly with many metals (such as Li, Ca, Mg) when heated to give ionic nitrides. Nitrogen is also easily converted to a liquid (b.p, -195.8°C) and can dissolve in water slightly. 14.1.2 The elemental substances of nitrogen family
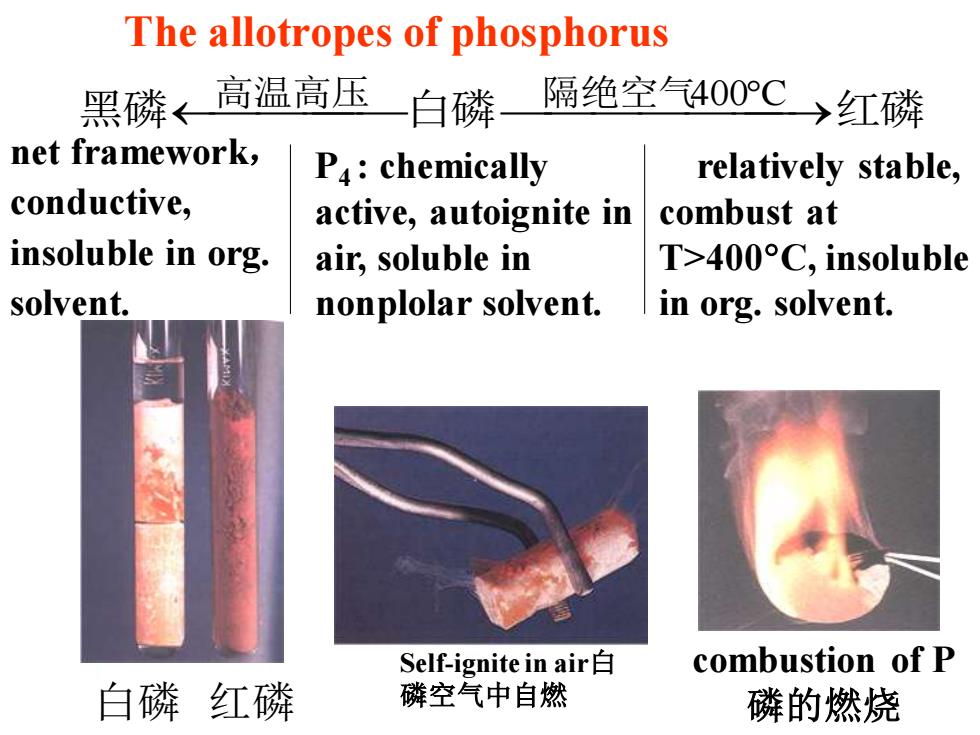
The allotropes of phosphorus 黑磷←高温高压 白磷 隔绝空气400C)红磷 net framework, P4:chemically relatively stable, conductive, active,autoignite in combust at insoluble in org. air,soluble in T>400°C,insoluble solvent. nonplolar solvent. in org.solvent. Self-ignite in air白 combustion of P 白磷红磷 磷空气中自燃 磷的燃烧
白磷 红磷 The allotropes of phosphorus 黑磷⎯高温高压 ⎯ ⎯⎯白磷⎯隔绝空气 ⎯⎯⎯⎯400 ⎯C →红磷 P4 : chemically active, autoignite in air, soluble in nonplolar solvent. relatively stable, combust at T>400C, insoluble in org. solvent. combustion of P 磷的燃烧 Self-ignite in air白 磷空气中自燃 net framework, conductive, insoluble in org. solvent

白磷的结构 红磷的结构 好时 Bi Sb 黑磷的结构 N2,P:metalloid, As,Sb:quasi-metal, Bi:metal
As Sb Bi N2 , P: metalloid, As, Sb: quasi-metal, Bi: metal 白 磷 的 结 构 红 磷 的 结 构 黑磷的结构

14.1.3 The compounds of nitrogen 1.Hydride of nitrogen ◆ ammonia (NH3) molecular N:sp3 hybridization,triangular structure: pyramid molecular structure N 100.8pm 107.30 H H H H
14.1.3 The compounds of nitrogen molecular structure: N:sp3 hybridization, triangular pyramid molecular structure 1. Hydride of nitrogen N H 107.3o .. H H ◆ ammonia (NH3 )

Preparation: In laboratory: 2NHCI+Ca(OH)2>CaCl2 +2H2O+2NH3(g) In industry process: N+3H2 450-500C 30MPa Fe >2NH Chemical properties: 1 Soluble in water forming monoprotic weak base NH+H,ONH·HO=NH+OH 2 Strong reducing ability 4NH3 +302(pure)>2N2 +6H2O 4NH,+50,a)24N0+6H,0
In industry process: 2NH Cl Ca(OH) CaCl 2H O 2NH (g) 4 + 2 ⎯→ 2 + 2 + 3 N2 3H2 2NH3 + ⎯450 ⎯⎯~500 ⎯ ⎯C 30MPa ⎯⎯ ⎯Fe→ Preparation: In laboratory: Chemical properties: ① Soluble in water forming monoprotic weak base ② Strong reducing ability _ NH3 +H2O NH3 H2O NH4 +OH + 4NH 5O (air) 4NO 6H O 4NH 3O (pure) 2N 6H O 2 P t 3 2 3 2 2 2 + ⎯⎯→ + + → + 800oc
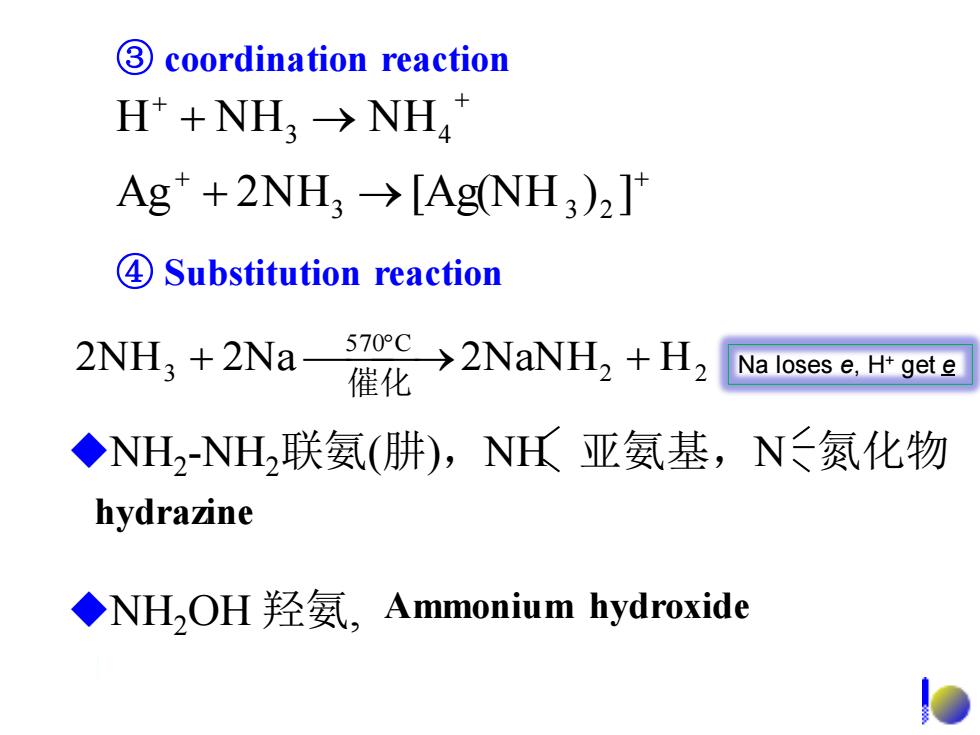
3 coordination reaction H+NH3→NH4 Ag+2NH3→[AgNH3)2] 4Substitution reaction 2NH3 +2Na- >2NaNH,H, Na loses e,H+gete ◆NH2-NH2联氨(肼),NH氏亚氨基,N≤氮化物 hydrazine ◆NH,OH羟氨,Ammonium hydroxide
④ Substitution reaction + + + + + → + → Ag 2N H [Ag(NH ) ] H N H N H 3 3 2 3 4 2 2 570 C 2NH3 + 2Na ⎯⎯⎯→2NaNH + H 催化 ③ coordination reaction ◆NH2 -NH2联氨(肼),NH 亚氨基,N 氮化物 hydrazine ◆NH2OH 羟氨, Ammonium hydroxide Na loses e, H+ get e
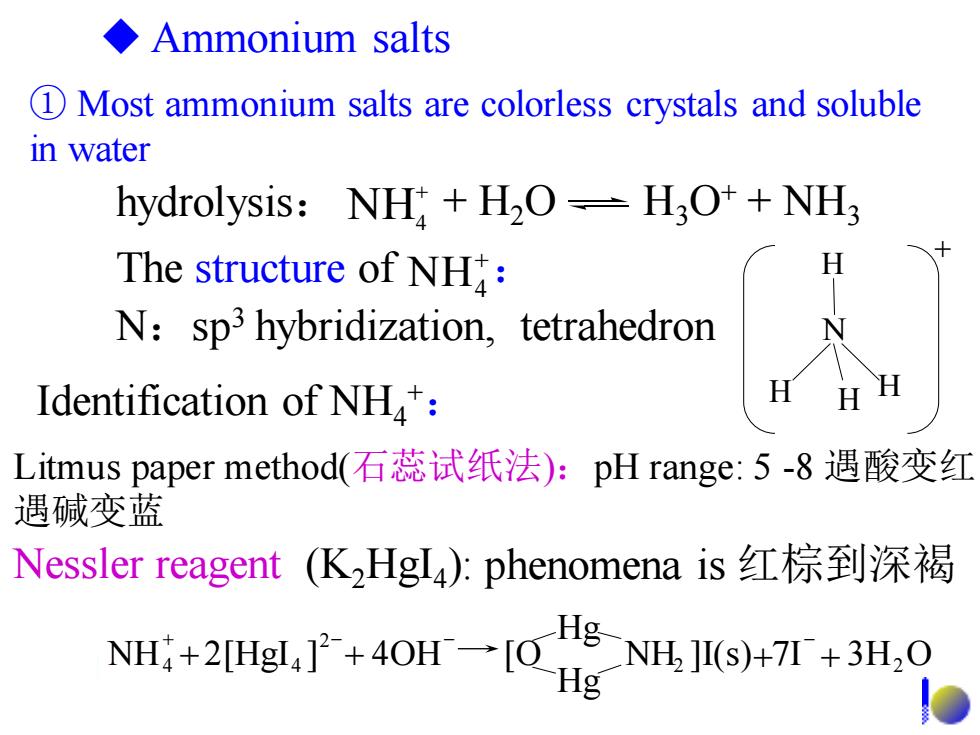
◆Ammonium salts 1 Most ammonium salts are colorless crystals and soluble in water hydrolysis:NH H2O=H3O++NH3 The structure of NH: N:sp3 hybridization,tetrahedron Identification of NH: H Litmus paper method(石蕊试纸法):pH range:5-8遇酸变红 遇碱变蓝 Nessler reagent(K2HgI4:phenomena is红棕到深褐 NH+2Hg,广+40H一OHg g-NH.HI(s)+I+3H.O
① Most ammonium salts are colorless crystals and soluble in water H N H H H + ◆ Ammonium salts Litmus paper method(石蕊试纸法):pH range: 5 -8 遇酸变红, 遇碱变蓝 + NH4 The structure of : Nessler reagent (K2HgI4 ): phenomena is 红棕到深褐 N:sp3 hybridization, tetrahedron Identification of NH4 +: NH ]I(s) 7I 3H O Hg Hg NH 2[HgI ] 4OH [O 2 2 2 4 + 4 + + + + - - - hydrolysis: + H2O H3O+ + NH3 + NH4