Chapter 17 The d-block elements(ID X$17.1 The elements of copper family x$17.2 The elements of zinc family
Chapter 17 The d-block elements( Ⅱ ) §17.1 The elements of copper family §17.2 The elements of zinc family

17.1 The elements of copper family >17.1.1 The elemental substances of copper family -17.1.2 The compounds of copper -17.1.3 The copper ions in aqueous solution and the related reactions
§17.1 The elements of copper family 17.1.1 The elemental substances of copper family 17.1.2 The compounds of copper 17.1.3 The copper ions in aqueous solution and the related reactions

17.1.1 The elemental substances of copper family 1.Existence Elemental substance:Cu,Ag,Au Mineral::malachite:Cu2(OHD)2CO3(孔雀石) argentite:Ag2S(辉银矿) calaverite:AuTe2(碲金矿) 2.Physical properties .Color:Cu(violet red),Ag(white),Au(yellow) *m.p and b.p are lower than that of other transition metals .Good conductor of heat and electricity,the order is Ag>Cu>Au .Good ductibility
17.1.1 The elemental substances of copper family Elemental substance :Cu, Ag, Au Mineral: malachite :Cu 2(OH) 2CO3 (孔雀石 ) argentite :Ag 2S (辉银矿) calaverite :AuTe2 (碲金矿 ) 1.Existence 2.Physical properties •Color :Cu(violet red), Ag(white), Au(yellow) •m.p and b.p are lower than that of other transition metals •Good conductor of heat and electricity, the order is Ag>Cu>Au •Good ductibility
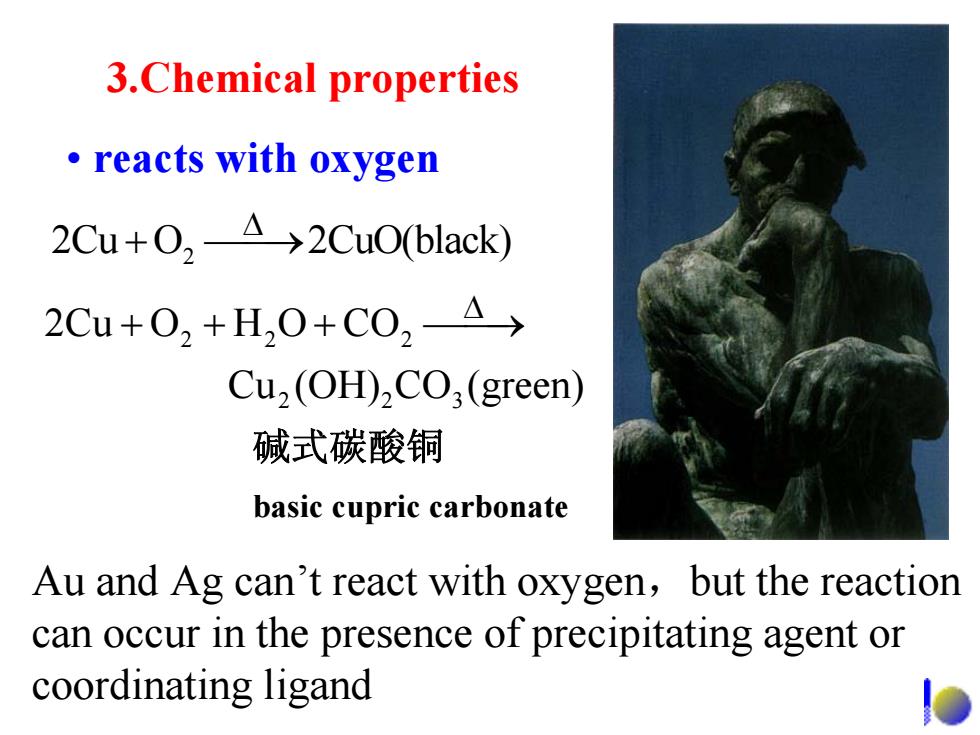
3.Chemical properties ·reacts with oxygen 2Cu+02A→2Cu0(black) 2Cu+02+H,0+C0,A Cu,(OH),CO;(green) 碱式碳酸铜 basic cupric carbonate Au and Ag can't react with oxygen,but the reaction can occur in the presence of precipitating agent or coordinating ligand
3.Chemical properties • reacts with oxygen 2 2Cu O 2CuO(black) Δ + ⎯⎯→ 碱式碳酸铜 basic cupric carbonate Au and Ag can’t react with oxygen ,but the reaction can occur in the presence of precipitating agent or coordinating ligand 22 2 2 23 2Cu O H O CO Cu (OH) CO (green) Δ + + + ⎯⎯→

-M=Cu,Ag,Au 4M+0,+2H,O+8CN->4[M(CN)2]+40H 4Cu+O,+2H,O+8NH3→ 4[CuNH3)2](无色)+4OH 02 colorless [Cu(NH3)4]2(蓝)blue Aqueous ammonia can't be shipped in copper containers. 2Ag+2H S+02->2AgS+2HO Silver turnS black after sever years
= AuAg,Cu,M )(])[Cu(NH 2 43 + 蓝 Aqueous ammonia can’t be shipped in copper containers. OS2H2Ag O2HS2Ag 22 ⎯++ ⎯→ 2 + 2 O2 Silver turnS black after sever years. + − + ⎯+++ ⎯→ OH4)(])4[Cu(NH 8NHO2HO4Cu 23 22 3 无色 − − − 8CNO2HO4M ⎯+++ ⎯→ + 4OH]4[M(CN) 22 2 blue colorless
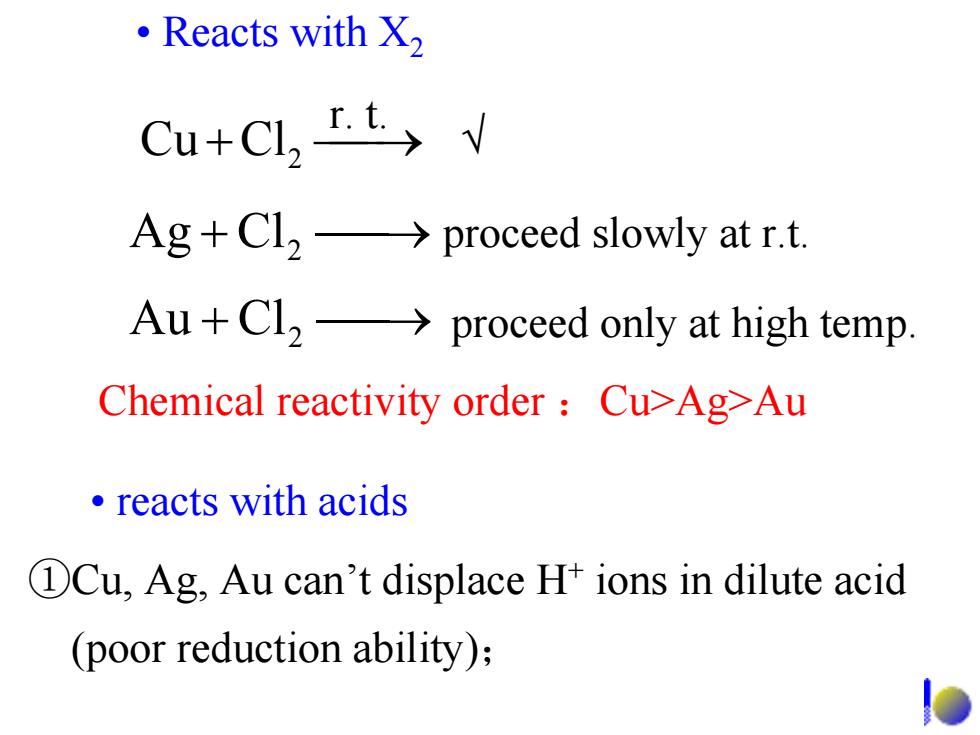
·Reacts with X) Cu+Cl,I.t> Ag+Cl2->proceed slowly at r.t. Au+Cl2->proceed only at high temp. Chemical reactivity order Cu>Ag-Au ·reacts with acids DCu,Ag,Au can't displace H+ions in dilute acid (poor reduction ability);
• Reacts with X2 ClCu 2 ⎯+ ⎯→r. t. Chemical reactivity order :Cu>Ag>Au ⎯+ ⎯→ proceed only at high temp. ClAu 2 ⎯+ ⎯→ proceed slowly at r.t. ClAg 2 • reacts with acids ①Cu, Ag, Au can’t displace H+ ions in dilute acid (poor reduction ability); √
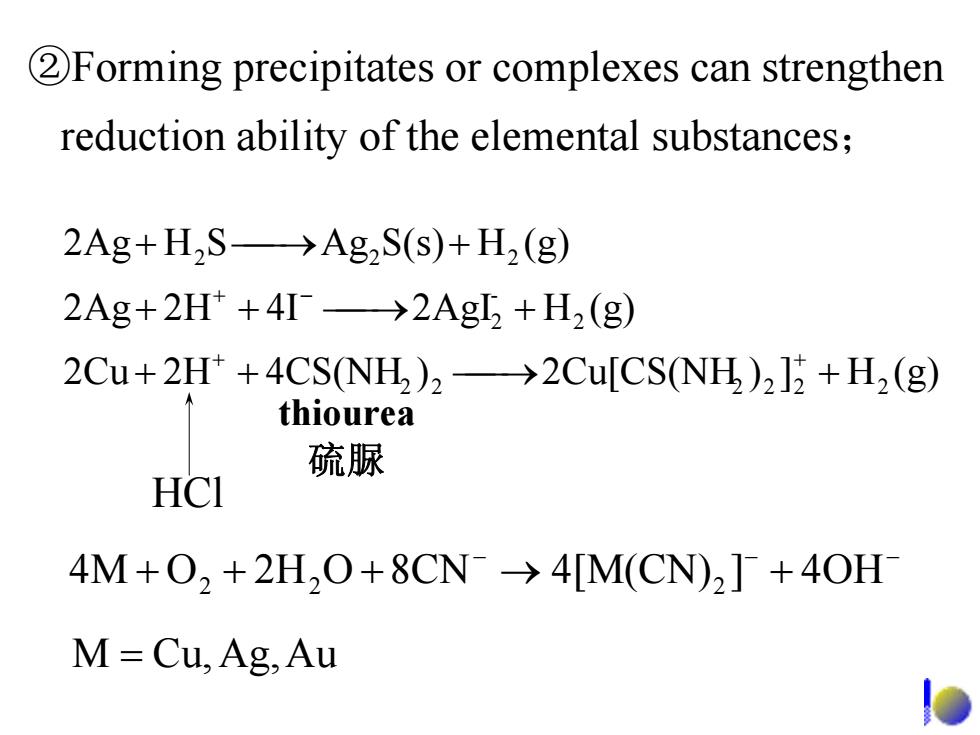
2Forming precipitates or complexes can strengthen reduction ability of the elemental substances; 2Ag+H2S->Ag2S(s)+H2(g) 2Ag+2H+4I→2Ag+H2(g) 2Cu+2H*+4CS(NH)2->2Cu[CS(NH)212 +H2(g) thiourea 硫脲 HCI 4M+0,+2H,O+8CN->4[M(CN),+40H M=Cu,Ag,Au
2H2Cu )4CS(NH 2Cu[CS(NH (g)H]) 4I2H2Ag (g)H2AgI (g)HS(s)AgSH2Ag 22 2222 2 - 2 2 2 2 ++ ⎯⎯→ + ⎯++ ⎯→ + ⎯+ ⎯→ + + + −+ HCl thiourea 硫脲 ②Forming precipitates or complexes can strengthen reduction ability of the elemental substances; − − − 8CNO2HO4M →+++ + 4OH]4[M(CN) 22 2 = AuAg,Cu,M
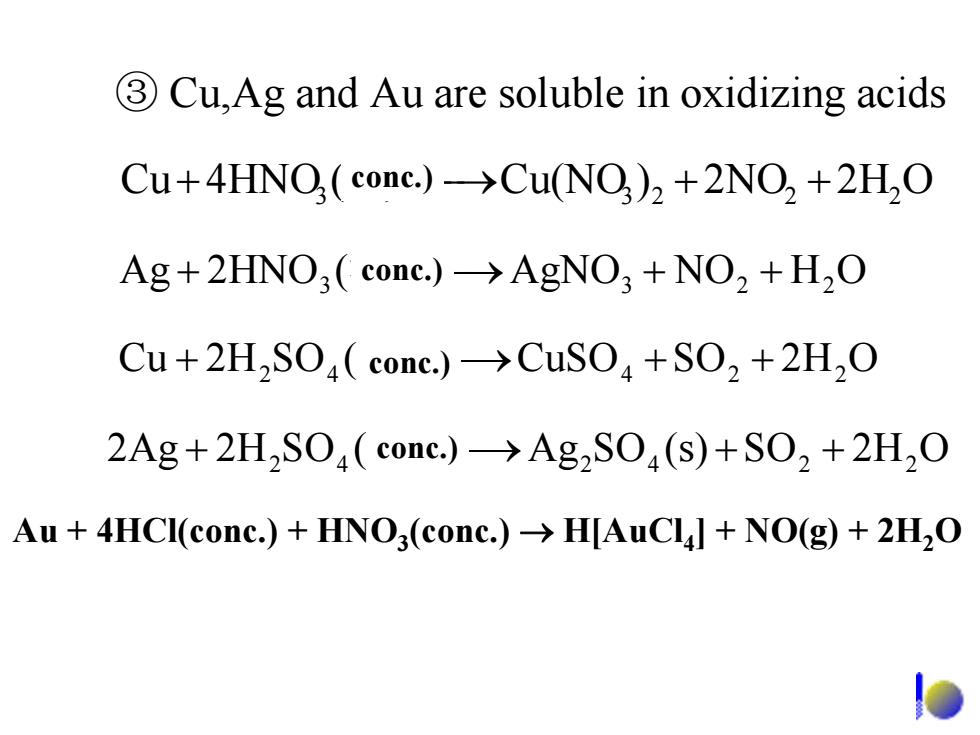
3Cu,Ag and Au are soluble in oxidizing acids Cu+4HNO(cone.)->Cu(NO)2+2NO2 +2H2O Ag+2HNO3 cone.)->AgNO3 +NO2 +H2O Cu+2H2SO(cone.)->CuSO+SO2+2H2O 2Ag+2H2SO(conc.)->Ag2SO(s)+SO2+2H2O Au 4HCI(conc.)+HNO3(conc.)>H[AuCll NO(g)+2H2O
③ Cu,Ag and Au are soluble in oxidizing acids + 3 浓)(4HNOCu ⎯⎯→ 23 ++ 22 O2H2NO)Cu(NO O2HSO(s)SOAg)(SO2H2Ag + 42 浓 ⎯⎯→ 42 ++ 22 + 42 浓)(SO2HCu ⎯⎯→CuSO ++ 224 O2HSO + 3 浓)(2HNOAg ⎯⎯→AgNO3 ++ 22 OHNO conc.) conc.) conc.) conc.) Au + 4HCl(conc.) + HNO3(conc.) → H[AuCl4] + NO(g) + 2H2O

17.1.2 The compounds of copper 1.The compounds of copper Cu(①):dlo,Compounds of Cu(①are white or colorless, no d-d transition Cu(⑩:d',Compounds of Cu(I)have colors, d-d transition Cu(IID):d8,K3CuF(light green),strong oxidizing agent d-d transition
1.The compounds of copper 17.1.2 The compounds of copper Cu(I): d10, Compounds of Cu(I) are white or colorless, no d-d transition Cu(II): d 9, Compounds of Cu(II) have colors, d-d transition Cu(III): d 8, K3CuF 6(light green), strong oxidizing agent d-d transition

17.1.2 The compounds of copper 1.The compounds of copper Cu⑩:do,Compounds of Cu⑩are white or colorless, no d-d transition Cu(ID:d9,Compounds of Cu(II)have colors, d-d transition Cu(IID):d3,K3CuF(light green),strong oxidizing agent d-d transition
1.The compounds of copper 17.1.2 The compounds of copper Cu(I): d10, Compounds of Cu(I) are white or colorless, no d-d transition Cu(II): d 9, Compounds of Cu(II) have colors, d-d transition Cu(III): d 8, K3CuF 6(light green), strong oxidizing agent d-d transition