
Chapter 2 Basic of thermodynamics To study chemistry using the theories and methods of thermodynamics 1)heat exchange in chemical reactions 2)directions and extent of reaction processes x 2.1 the First law of thermodynamics §2.2 Thermochemistry x 2.3 Direction of a chemical reaction
Chapter 2 Basic of thermodynamics §2.2 Thermochemistry §2.3 Direction of a chemical reaction To study chemistry using the theories and methods of thermodynamics 1) heat exchange in chemical reactions 2) directions and extent of reaction processes §2.1 the First law of thermodynamics

2.1 the First law of thermodynamics 2.1.1 Basic terms in thermodynamics 1.System and surroundings 2.State and state functions 3.Process and path 4.Volume work 5.Thermodynamic energy 2.1.2 the First law of thermodynamics 1.the Content of the First law of thermodynamics 2.Work and heat
2.1.1 Basic terms in thermodynamics 1. System and surroundings 5. Thermodynamic energy 4. Volume work 3. Process and path 2. State and state functions §2.1 the First law of thermodynamics 2.1.2 the First law of thermodynamics 1. the Content of the First law of thermodynamics 2. Work and heat

2.1 the First law of thermodynamics 2.1.1 Basic terms in thermodynamics 1.System and surroundings 2.State and state functions 3.Process and path 4.Volume work 5.Thermodynamic energy 2.1.2 the First law of thermodynamics 1.the Content of the First law of thermodynamics 2.Work and heat
2.1.1 Basic terms in thermodynamics 1. System and surroundings 5. Thermodynamic energy 4. Volume work 3. Process and path 2. State and state functions §2.1 the First law of thermodynamics 2.1.2 the First law of thermodynamics 1. the Content of the First law of thermodynamics 2. Work and heat
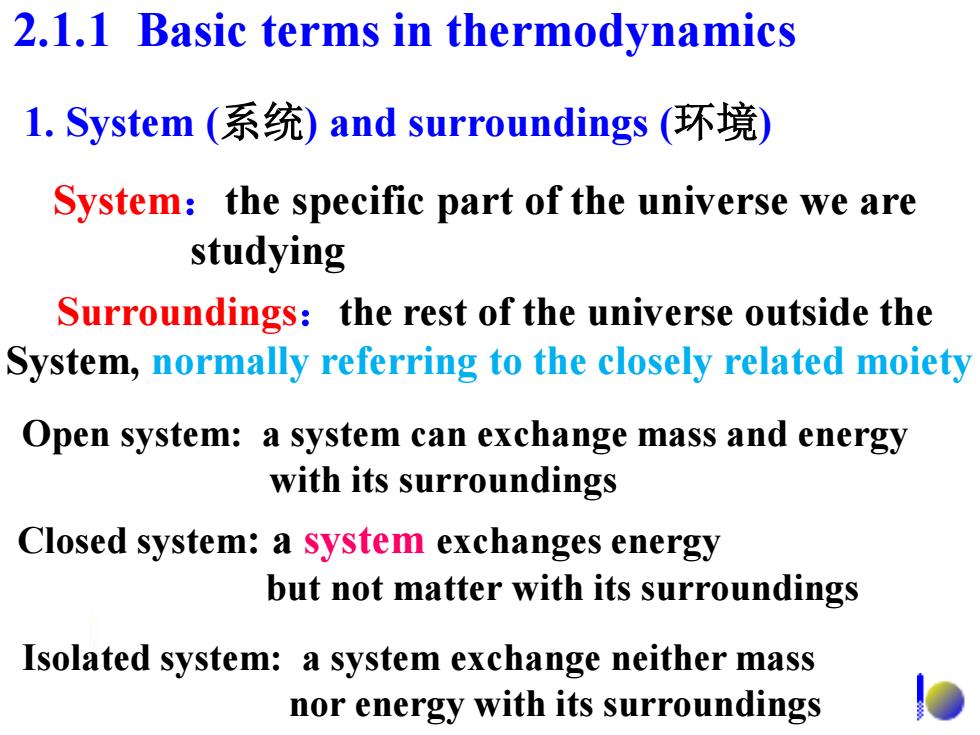
2.1.1 Basic terms in thermodynamics l.System(系统)and surroundings(环境) System:the specific part of the universe we are studying Surroundings:the rest of the universe outside the System,normally referring to the closely related moiety Open system:a system can exchange mass and energy with its surroundings Closed system:a system exchanges energy but not matter with its surroundings Isolated system:a system exchange neither mass nor energy with its surroundings
System:the specific part of the universe we are studying Surroundings:the rest of the universe outside the System, normally referring to the closely related moiety Open system: a system can exchange mass and energy with its surroundings Closed system: a system exchanges energy but not matter with its surroundings Isolated system: a system exchange neither mass nor energy with its surroundings 2.1.1 Basic terms in thermodynamics 1. System (系统) and surroundings (环境)

Three systems represented by water in a flask (a) (b) (c)
Three systems represented by water in a flask (a) (b) (c)
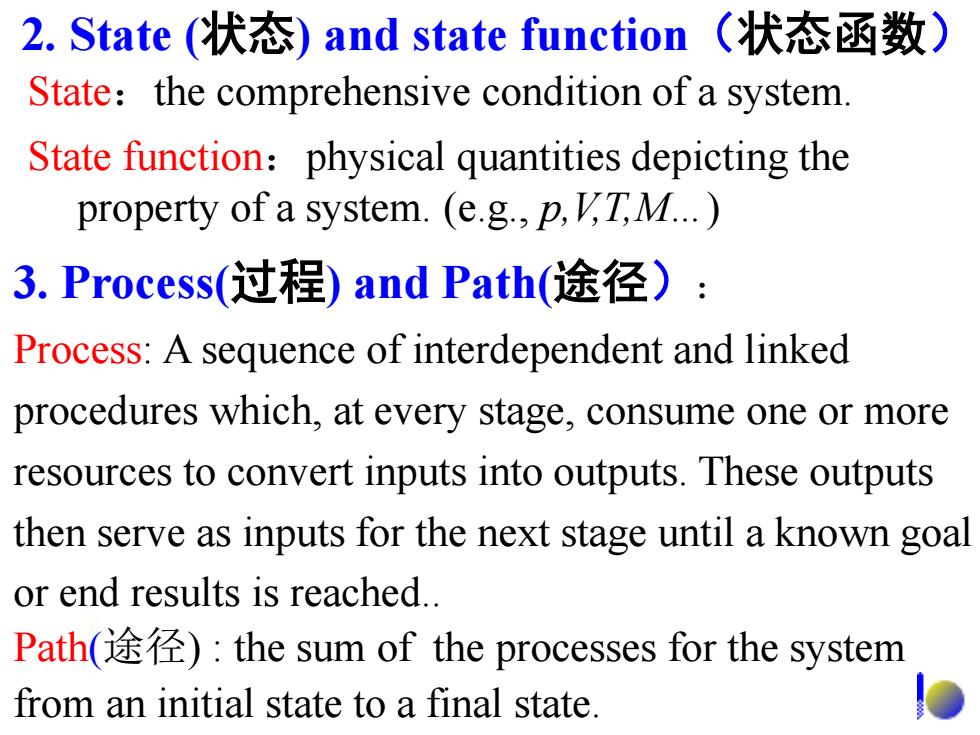
2.State(状态)and state function(状态函数) State:the comprehensive condition of a system. State function:physical quantities depicting the property of a system.(e.g.,p,V'T,M...) 3.Process(过程)and Path(途径): Process:A sequence of interdependent and linked procedures which,at every stage,consume one or more resources to convert inputs into outputs.These outputs then serve as inputs for the next stage until a known goal or end results is reached.. Path(途径):the sum of the processes for the system from an initial state to a final state
2. State (状态) and state function(状态函数) State:the comprehensive condition of a system. State function:physical quantities depicting the property of a system. (e.g., p,V,T,M…) 3. Process(过程) and Path(途径): Process: A sequence of interdependent and linked procedures which, at every stage, consume one or more resources to convert inputs into outputs. These outputs then serve as inputs for the next stage until a known goal or end results is reached.. Path(途径) : the sum of the processes for the system from an initial state to a final state

Path/process (I) Final state Initial state Path/process (II) The characteristics of a state function: (1)the value of a state function is unique when the state of a system is specified. (2)the change in the value of a state function depends only on an initial state and a final state of a system and is independent of the path(途径) /process(过程)between the two states
(2) the change in the value of a state function depends only on an initial state and a final state of a system and is independent of the path (途径) /process(过程) between the two states. Initial state Final state Path/process (II) Path/process (I) The characteristics of a state function: (1) the value of a state function is unique when the state of a system is specified
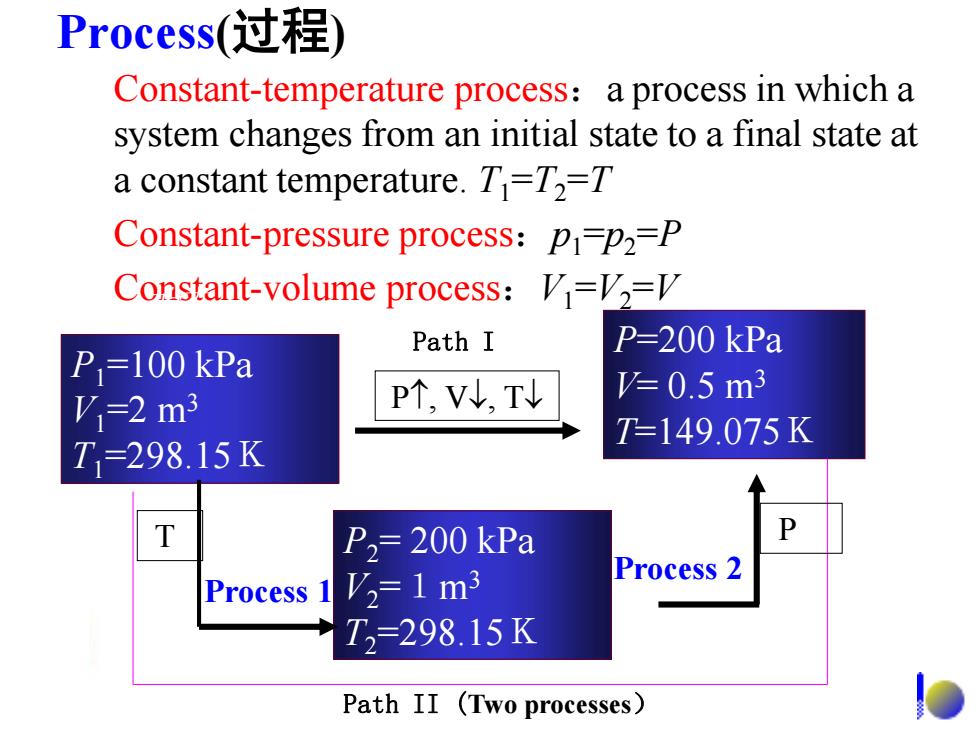
Process(过程 Constant-temperature process:a process in which a system changes from an initial state to a final state at a constant temperature.T=T2=T Constant-pressure process:pi=p2=P Constant-volume process:V=12= Path I P=200 kPa P=100 kPa P个,V,T =0.5m3 V=2m3 T=149.075K T=298.15K P2=200 kPa P V2=1m3 Process 2 Process 1 T2=298.15K Path II (Two processes)
Process(过程) Constant-temperature process:a process in which a system changes from an initial state to a final state at a constant temperature. T1 =T2 =T Constant-pressure process:p1 =p2 =P Constant-volume process:V1 =V2 =V P1=100 kPa V1=2 m3 T1=298.15K P2= 200 kPa V2 =1m3 T2=298.15K P, V, T P=200 kPa V= 0.5 m3 T=149.075K 始态 Path II (Two processes) Path I Process 1 T Process 2 P
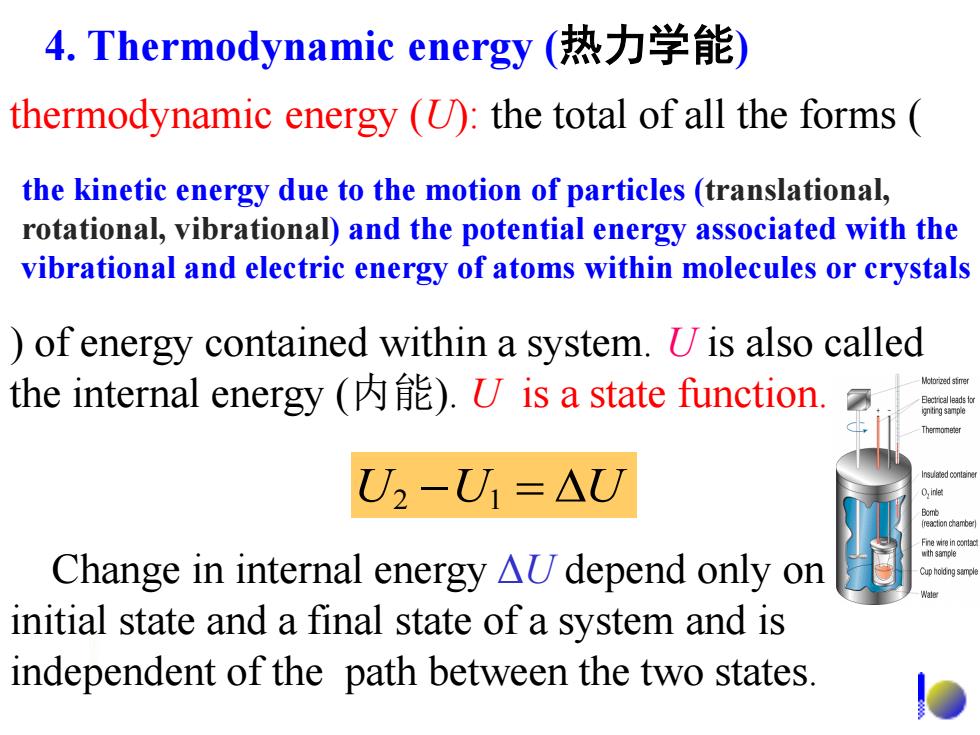
4.Thermodynamic energy(热力学能) thermodynamic energy (U):the total of all the forms the kinetic energy due to the motion of particles(translational, rotational,vibrational)and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals )of energy contained within a system.U is also called the internal energy )U is a state function. Molorined stimer U2-U1=△U hs别n 0,e eatonctarte Change in internal energy aU depend only on initial state and a final state of a system and is independent of the path between the two states
thermodynamic energy (U): the total of all the forms ( ) of energy contained within a system. U is also called the internal energy (内能). U is a state function. U2 U1 U Change in internal energy ΔU depend only on an initial state and a final state of a system and is independent of the path between the two states. the kinetic energy due to the motion of particles (translational, rotational, vibrational) and the potential energy associated with the vibrational and electric energy of atoms within molecules or crystals 4. Thermodynamic energy (热力学能)
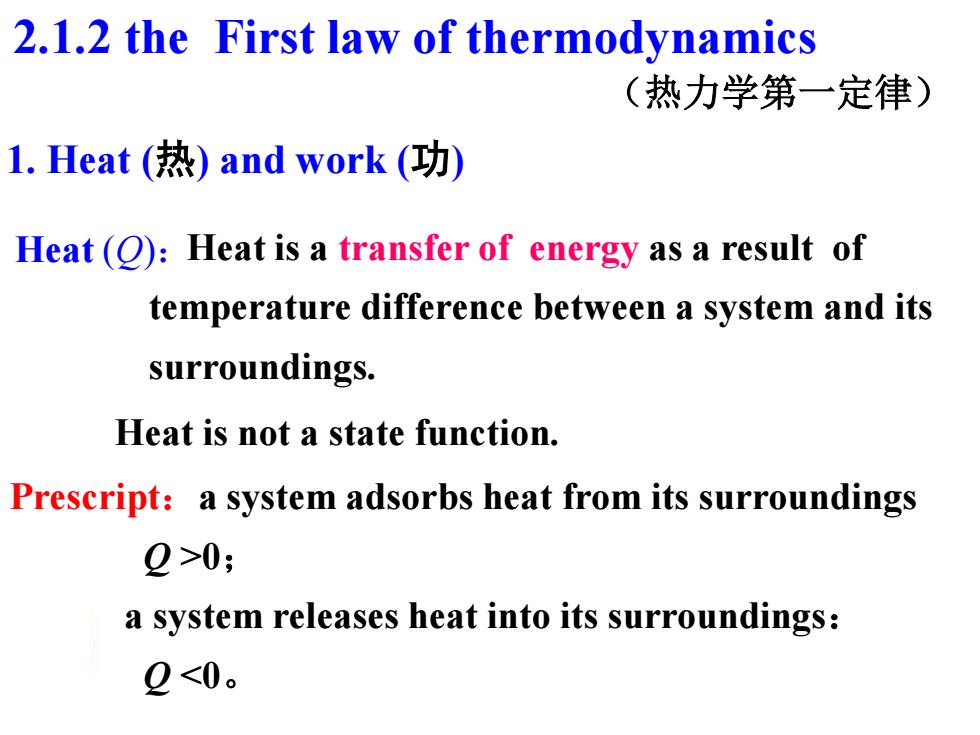
2.1.2 the First law of thermodynamics (热力学第一定律) l.Heat(热)and work(功) Heat():Heat is a transfer of energy as a result of temperature difference between a system and its surroundings. Heat is not a state function. Prescript:a system adsorbs heat from its surroundings 2>0; a system releases heat into its surroundings: 2<0
1. Heat (热) and work (功) Heat is a transfer of energy as a result of temperature difference between a system and its surroundings. Heat (Q): Heat is not a state function. Prescript:a system adsorbs heat from its surroundings Q >0; a system releases heat into its surroundings: Q <0。 2.1.2 the First law of thermodynamics (热力学第一定律)