
21 The elements of chromium and Manganese family 21.1 The elemental substances of Cr,Mo and W 21.2 The compounds of chromium 21.3 The elemental substance of Mn -21.4 The compounds of manganese
21.2 The compounds of chromium 21.1 The elemental substances of Cr, Mo and W §21 The elements of chromium and Manganese family 21.3 The elemental substance of Mn 21.4 The compounds of manganese

21.1 The elemental substances of Cr, Mo and W Group VIB elements:Cr,Mo,W Valence electron configuration: Cr,Mo:(n-1)d5ns1,W:5d46s2 1.The preparation of elemental Cr (the hardest metal It exists in the form of chromite Fe(CrO2)2 (铬铁矿)
Group VIB elements:Cr, Mo, W It exists in the form of chromite Fe(CrO2 )2 (铬铁矿) 1. The preparation of elemental Cr (the hardest metal ) 21.1 The elemental substances of Cr, Mo and W Valence electron configuration: Cr, Mo: (n-1)d5ns1 , W: 5d46s2
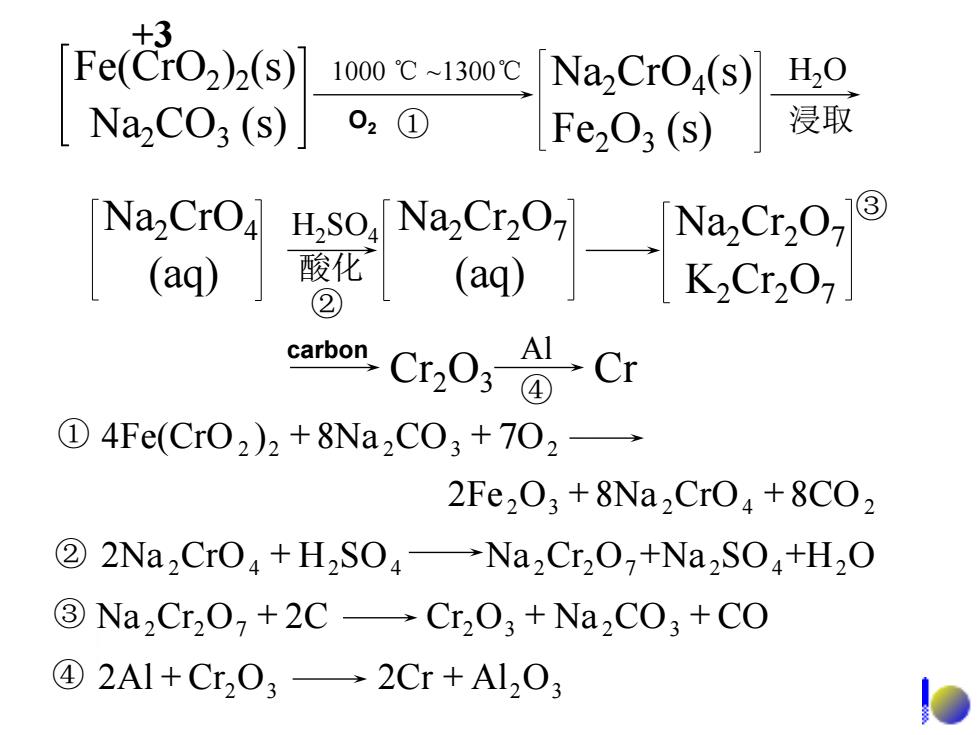
+3 Fe(CrO2)2(s) 1000℃~1300℃ Na,CrOa(s) H20 NazCO3(s) 02① Fe203(s) 浸取 Na2CrO4 ·8 H2S04 Na2Cr2O 7③ (aq) ② carbon( ①4Fe(Cr02)2+8Na2C03+702 2Fe203+8Na2Cr04+8C02 ②2Na2CrO4+H2S04→Na2Cr202+Na2S04+H2O 3Na,Cr2O+2C-Cr2O3+Na2CO3+CO ④2Al+Cr203→2Cr+Al203
1000 ℃ ~1300℃ ① H2O 浸取 H2SO4 酸化 ② Cr2O3 Cr Al ④ Fe(CrO2 )2 (s) Na2CO3 (s) Na2CrO4 (s) Fe2O3 (s) Na2CrO4 (aq) Na2Cr2O7 (aq) Na2Cr2O7 K2Cr2O7 ③ 2Fe 2O3 8Na 2CrO4 8CO2 + + 2 2 8Na 2CO3 7O2 ① 4Fe(CrO ) + + ② 2Na 2CrO4 + H2SO4 Na 2Cr2O7 +Na 2SO4 +H2O 2Al Cr2O3 2Cr Al2O3 ④ + + ③ Na 2Cr2O7 + 2C Cr2O3 + Na 2CO3 + CO +3 O2 carbon
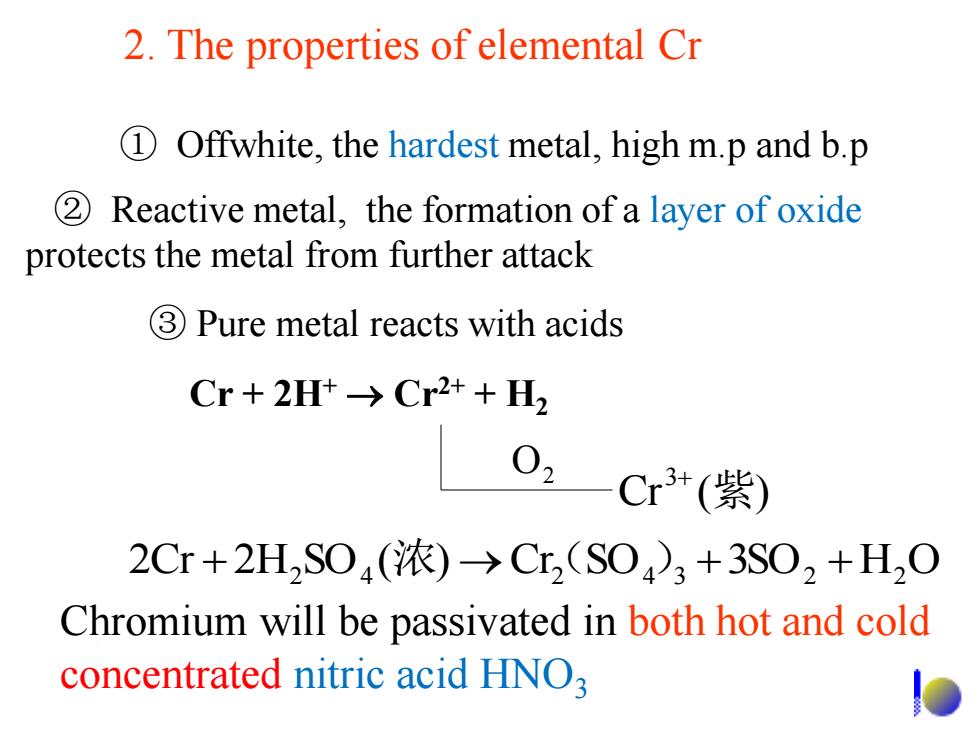
2.The properties of elemental Cr 1 Offwhite,the hardest metal,high m.p and b.p 2 Reactive metal,the formation of a layer of oxide protects the metal from further attack 3Pure metal reacts with acids Cr+2Ht→Cr2++H2 02 Cr3+(紫) 2Cr+2HS04(浓)→Cr2(S04)3+3S02+H2O Chromium will be passivated in both hot and cold concentrated nitric acid HNO
Chromium will be passivated in both hot and cold concentrated nitric acid HNO3 ③ Pure metal reacts with acids ② Reactive metal, the formation of a layer of oxide protects the metal from further attack ① Offwhite, the hardest metal, high m.p and b.p Cr ( ) 2 3+ 紫 O 2Cr + 2H2 SO4 (浓) Cr(2 SO4 )3 +3SO2 + H2 O Cr + 2H+ Cr2+ + H2 2. The properties of elemental Cr
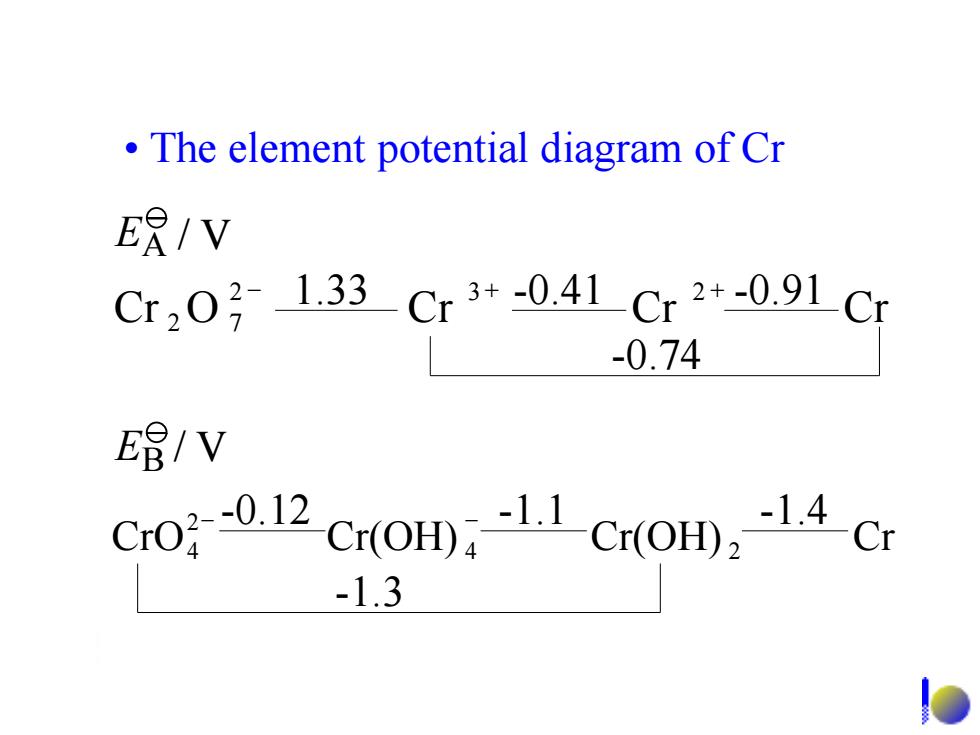
The element potential diagram of Cr ER/V Cr2031.33Cr3+-041Cr2+-0.91Cr -0.74 E唱/V Cro01 Cr(OH)Cr(OB),14-Cr -1.3
• The element potential diagram of Cr Cr O Cr Cr Cr 2 3 2 2 7 - + + -0.74 1.33 -0.41 -0.91 CrO Cr(OH) Cr(OH) Cr 4 2 2 4 - -0.12 - -1.1 -1.4 -1.3 EB / V EA / V

21.2 The compounds of chromium CrO3(铬绿) color m.p./℃ changes when heated 暗红色 250℃分解为 CrO3(铬酐) 198 Cr203与02 K,CrO 黄色 975 熔融不分解 K,Cr02(红矾) 橙红色 398 熔融不分解 Cr,O3(铬绿) 绿色 2330 不分解 CrCl 6H2O 紫色 83 失去结晶水 KCr(SO)2 12H2O 暗紫色 89 失去结晶水
color m.p./℃ changes when heated CrO3 (铬酐) 暗红色 198 250℃分解为 Cr2O3与O2 K2CrO4 黄色 975 熔融不分解 K2Cr2O7 (红矾) 橙红色 398 熔融不分解 Cr2O3 (铬绿) 绿色 2330 不分解 CrCl3·6H2O 紫色 83 失去结晶水 KCr(SO4 )2·12H2O 暗紫色 89 失去结晶水 Cr2O3 (铬绿) 21.2 The compounds of chromium

C,02 Different ions of Cr in aqueous solution color pH for their existence 3 Cr,03 橙红 6 Cr3+(aq) 紫 酸性 Cr(OH) 亮绿 强碱 Cr2+(aq)Cr3+(aq) Cr2+(aq) 蓝 酸性
color pH for their existence 橙红 <2 黄 >6 Cr3+(aq) 紫 酸性 亮绿 强碱 Cr2+(aq) 蓝 酸性 Different ions of Cr in aqueous solution 2- Cr2 O7 2- CrO4 - Cr(OH)4 Cr2+(aq) Cr3+(aq) 2- Cr2 O7 2- CrO4
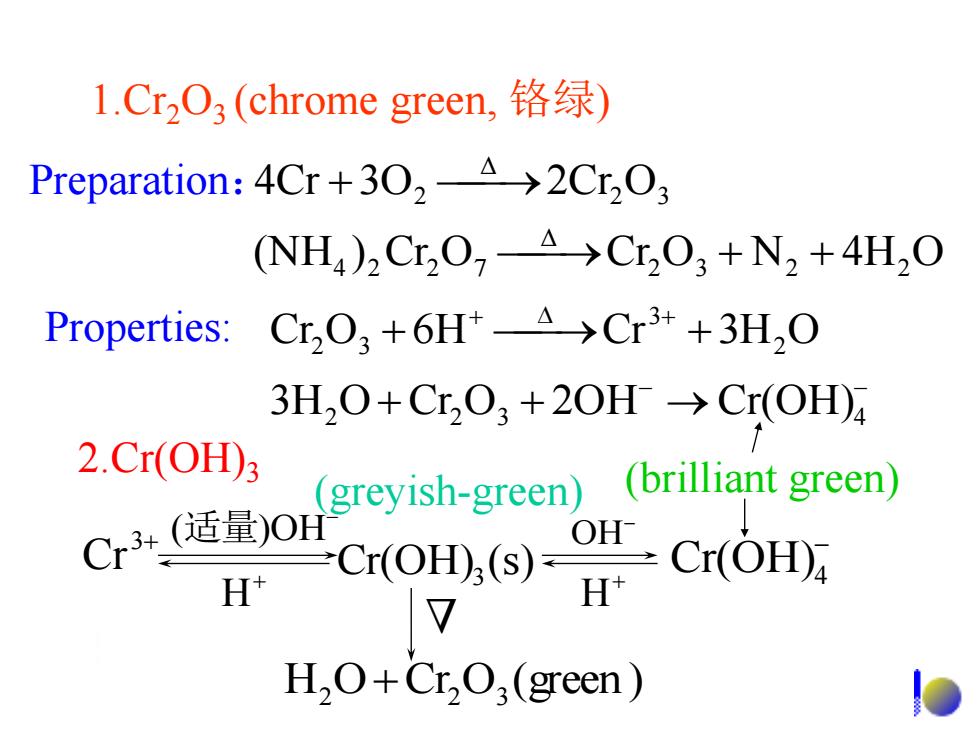
1.CrO3(chrome green,铬绿) Preparation:4Cr+302-A>2Cr2O3 (NH)2Cr2O7-A >Cr2O;+N2+4H2O Properties:Cr,O,+6H*A>Cr3++3H,O 3H2O+Cr2O3 +20H>Cr(OH) 2.Cr(OH)3 Cr3适量到)0H greyish-green)(brilliant green) H rot),) H2O+Cr,O(green)
Preparation: Properties: (NH ) Cr O Cr O N 4H O 4Cr 3O 2Cr O 2 3 2 2 Δ 4 2 2 7 2 3 Δ 2 + + + - - + + + + + + 2 2 3 4 2 3 2 3 3H O Cr O 2OH Cr(OH) Cr O 6H Cr 3H O 3+ Cr - Cr(OH)4 Cr(OH) (s) 3 H O Cr O (green ) 2 + 2 3 - (适量)OH - OH + H (greyish-green) (brilliant green) Δ 1.Cr2O3 (chrome green, 铬绿) 2.Cr(OH)3 + H
3.Salts of Cr(IID)ion Cr,(SO3,KCr(SO),12HO,CrCl Hydrolysis [Cr(H2O)]-[Cr(OH)(HO)3]+H K9H104 2[Cr(H2O)6]3t=[(H2O)4Cr(OH)2Cr(H2O)4]4+ 水合Cr离子双聚体 +2H+ 2Cr3++3S2-+6H,0→Cr(OH)3(S)+3H2S(g) 2Cr3++3C03+3H20→2Cr(OH),(s)+3C02(g)
2 4 3 4 2 2O, CrCl3 Cr(SO),KCr(SO)12H 2Cr 3CO 3H O 2Cr(OH (s) 3CO (g) 2Cr 3S 6H O Cr(OH (s) 3H S(g) 2 3 2 2- 3 3 2 3 2 3 2 + + + + + + + + - ) ) 3. Salts of Cr(Ⅲ) ion • Hydrolysis + + + [Cr(H O ] [Cr(OH)(H O ] + H 2 2 5 3 2 )6 ) 4 10- K 2[Cr(H2O)6 ] 3+ [(H2O)4 Cr(OH)2Cr(H2O)4 ] 4+ 水合Cr离子双聚体 +2H+
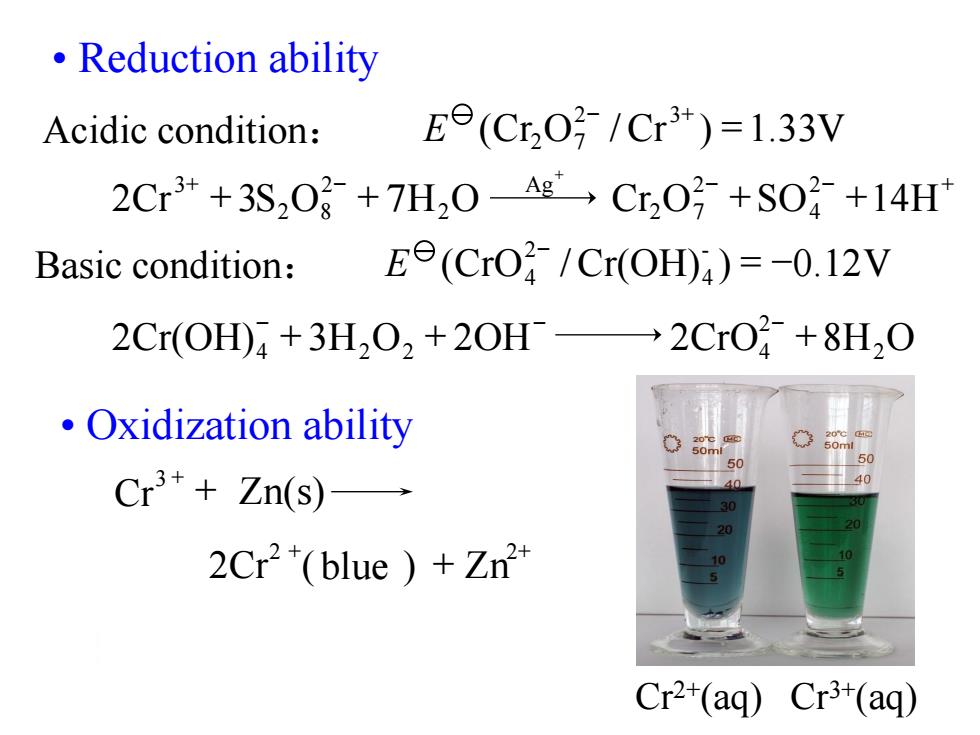
·Reduction ability Acidic condition: Ee(Cr,03/Cr3*)=1.33V 2Cr*+3S2O+7H2O-Ag Cr2O+SO+14H Basic condition: E(CrO/Cr(OH))=-0.12V 2Cr(OH)+3H2O2+20H-2CrO+8H,O Oxidization ability Cr3++Zn(s)- 2Cr"(blue Zn2 Cr2+(aq)Cr3+(aq)
• Reduction ability • Oxidization ability 2Cr ( blue ) Zn 2 + 2+ + Cr Zn(s) 3 + + 2Cr(OH) 3H O 2OH 2CrO 8H2O 2 4 2 2 4 + + + - - - 2Cr 3S O 7H O Cr O SO 14H 2 4 2 2 7 Ag 2 2 2 8 3 + + + + + - - - + + (Cr O /Cr ) 1.33V 2 3 2 7 = - + Acidic condition: E (CrO /Cr(OH) ) 0.12V - 4 2 4 = - - Basic condition: E Cr2+(aq) Cr3+(aq)