
14 The elements of carbon family 14.1 Outline on carbon family 14.2 The elemental substances of carbon family -14.3 The compounds of carbon 14.4 The compounds of silicon 14.5 The compounds of tin and lead
§ 14 The elements of carbon family 14.5 The compounds of tin and lead 14.4 The compounds of silicon 14.3 The compounds of carbon 14.2 The elemental substances of carbon family 14.1 Outline on carbon family
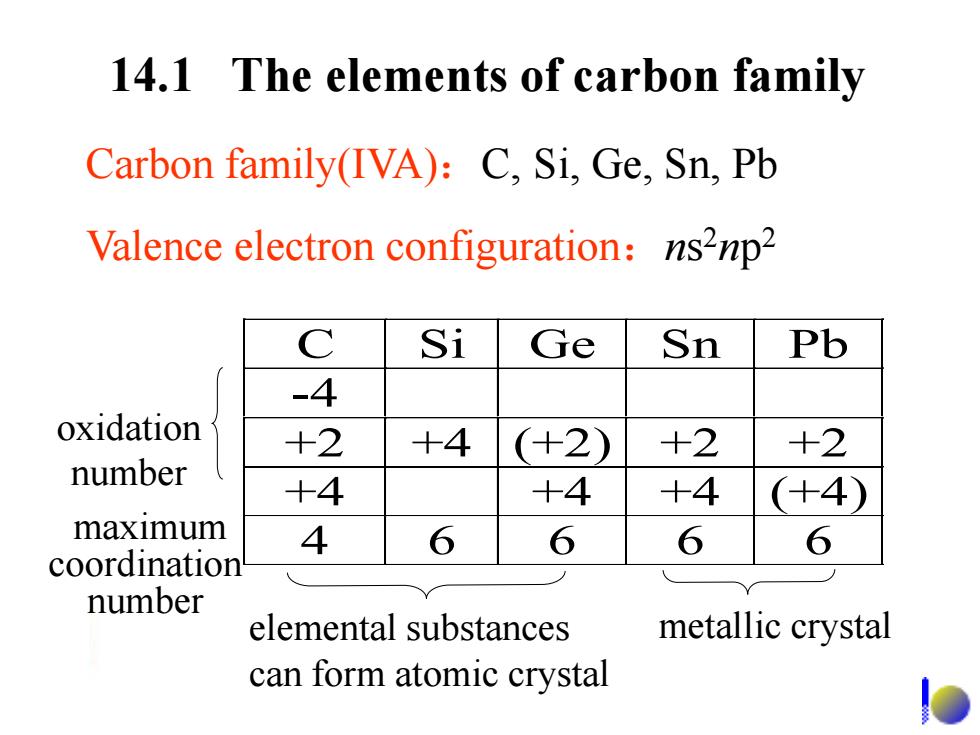
14.1 The elements of carbon family Carbon family(IVA):C,Si,Ge,Sn,Pb Valence electron configuration:ns2np2 C Si Ge Sn Pb -4 oxidation +2 +4 (+2) +2 +2 number +4 +4 +4 (+4) maximum 4 6 6 6 6 coordination number elemental substances metallic crystal can form atomic crystal
14.1 The elements of carbon family Valence electron configuration:ns 2np 2 C Si Ge Sn Pb -4 +2 +4 (+2) +2 +2 +4 +4 +4 (+4) 4 6 6 6 6 oxidation number maximum coordination number elemental substances can form atomic crystal metallic crystal Carbon family(IVA):C, Si, Ge, Sn, Pb
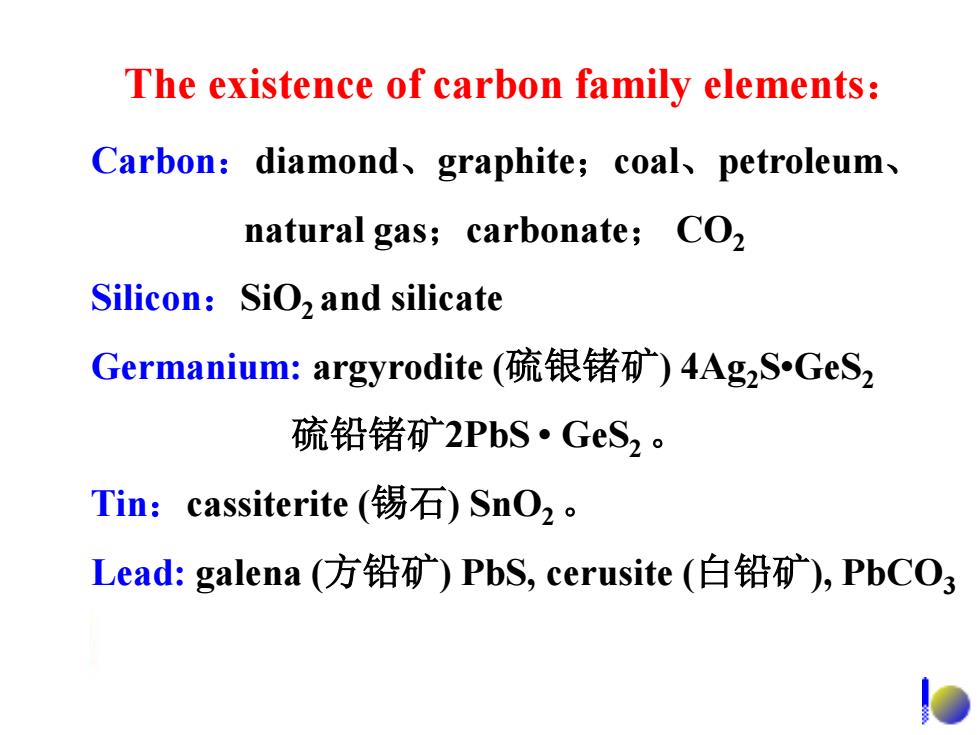
The existence of carbon family elements: Carbon:diamond、graphite;coal、petroleum、 natural gas;carbonate;CO2 Silicon:SiO2 and silicate Germanium:argyrodite(疏银锗矿)4AgzS.GeS2 疏铅锗矿2PbS·GeS2。 Tin:cassiterite(锡石)SnO2。 Lead:galena(方铅矿)PbS,cerusite(白铅矿),PbCO3 o
The existence of carbon family elements: Carbon:diamond、graphite;coal、petroleum、 natural gas;carbonate; CO2 Silicon:SiO2 and silicate Germanium: argyrodite (硫银锗矿) 4Ag2S•GeS2 硫铅锗矿2PbS • GeS2 。 Tin:cassiterite (锡石) SnO2 。 Lead: galena (方铅矿) PbS, cerusite (白铅矿), PbCO3

锡石Sn02 方铅矿PbS
锡石SnO2 方铅矿 PbS

14.2 The elemental substances of carbon family The allotropes of carbon in elementary form: (同素异形体) diamond:原子晶体,硬度最大,熔点最高 graphite:层状晶体,质软,有金属光泽 fullerenes:C60,C7o etc. C60是1985年用激光轰击石墨作碳的气化实验 时发现的。 C tubes: Graphene ●。000 碳纤维
The allotropes of carbon in elementary form: •diamond:原子晶体,硬度最大, 熔点最高。 •graphite:层状晶体 ,质软,有金属光泽。 •fullerenes: C60, C70 etc. C60 是1985年用激光轰击石墨作碳的气化实验 时发现的。 •C tubes: •Graphene •。。。。 碳纤维 (同素异形体) 14.2 The elemental substances of carbon family
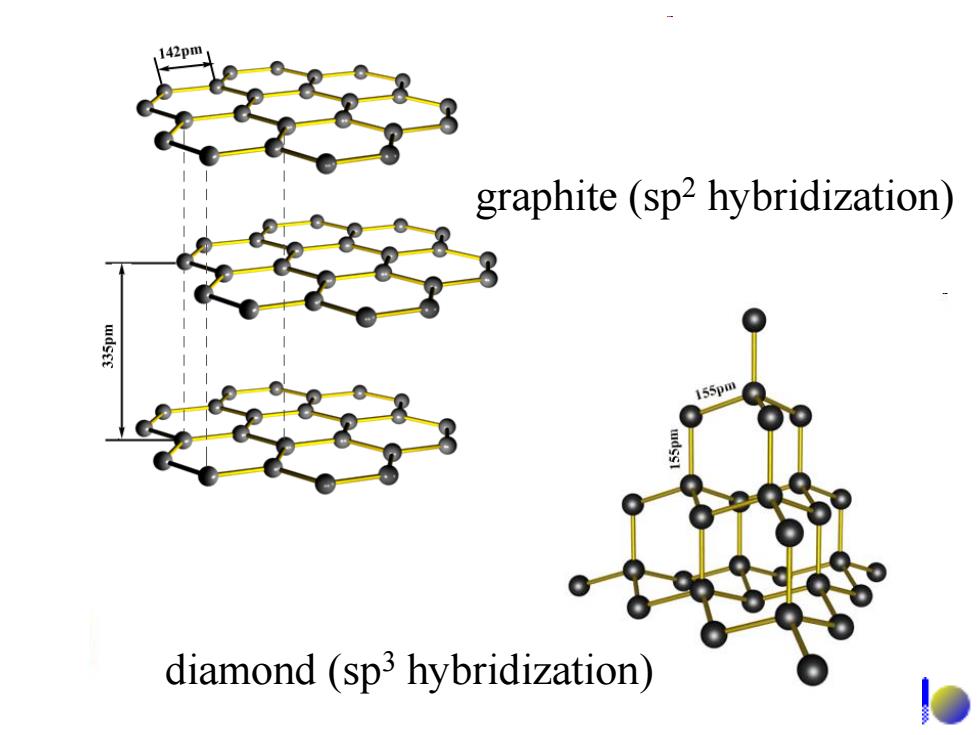
graphite(sp2 hybridization) 155pm diamond (sp3 hybridization)
graphite (sp2 hybridization) diamond (sp3 hybridization)
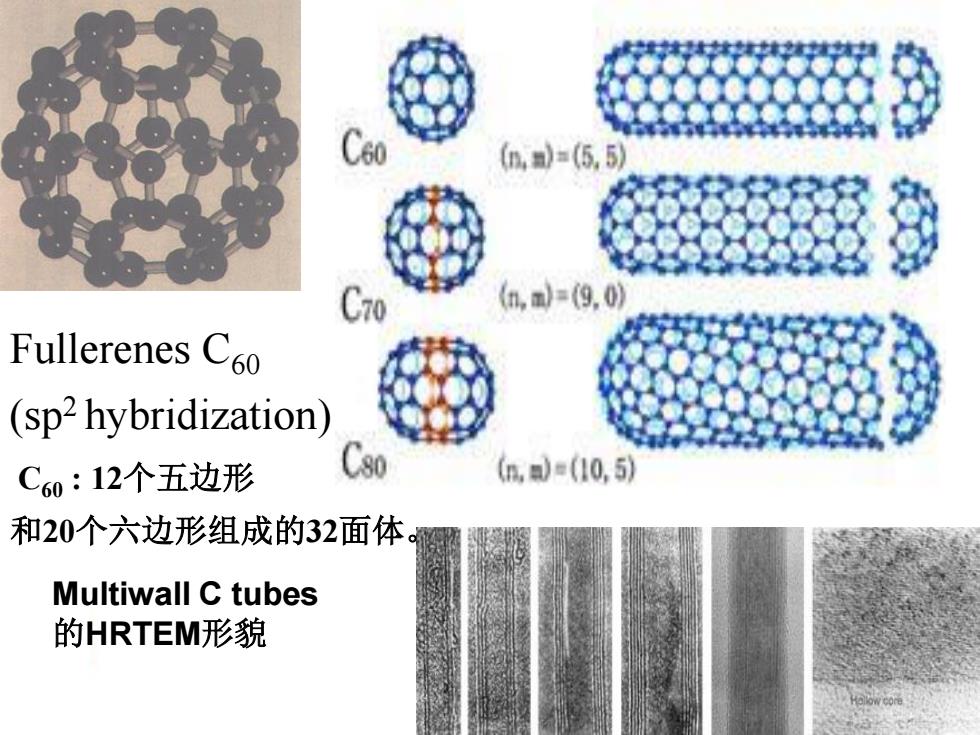
n,=(5,5 (h,=(9,0 Fullerenes C60 (sp2hybridization) C60:12个五边形 90 (,=(10,5 和20个六边形组成的32面体 Multiwall C tubes 的HRTEM形貌
Fullerenes C60 (sp2 hybridization) Multiwall C tubes 的HRTEM形貌 C60 : 12个五边形 和20个六边形组成的32面体

Silicon occurs as either amorphous structure or crystal,the structure of crystal is similar to that of diamond. Germanium is offwhite(灰白色)metal,.the structure of germanium is similar to that of diamond Tin occurs as the allotropes of grey tin,white tin and brittle tin: grey tin 13.white tin 161℃ brittle tin Lead is soft metal and can prevent substances from X-ray
Silicon occurs as either amorphous structure or crystal, the structure of crystal is similar to that of diamond. Germanium is offwhite(灰白色) metal, the structure of germanium is similar to that of diamond. Tin occurs as the allotropes of grey tin, white tin and brittle tin: Lead is soft metal and can prevent substances from X-ray. grey tin 13.2℃ white tin 161℃ brittle tin
14.3 The compounds of carbon 1.The oxides of carbon Carbon monoxide (CO) Structure features: C:2s22p2;0:2s22p4 C0(6+8=14e)andN2(2×7=14e)are isoelectronic substances,so they have similar structure
14.3 The compounds of carbon Structure features: CO(6+8=14e- ) and N2 (2×7=14e- ) are isoelectronic substances, so they have similar structure. 1.The oxides of carbon • Carbon monoxide (CO) C: 2s22p2 ; O: 2s22p4
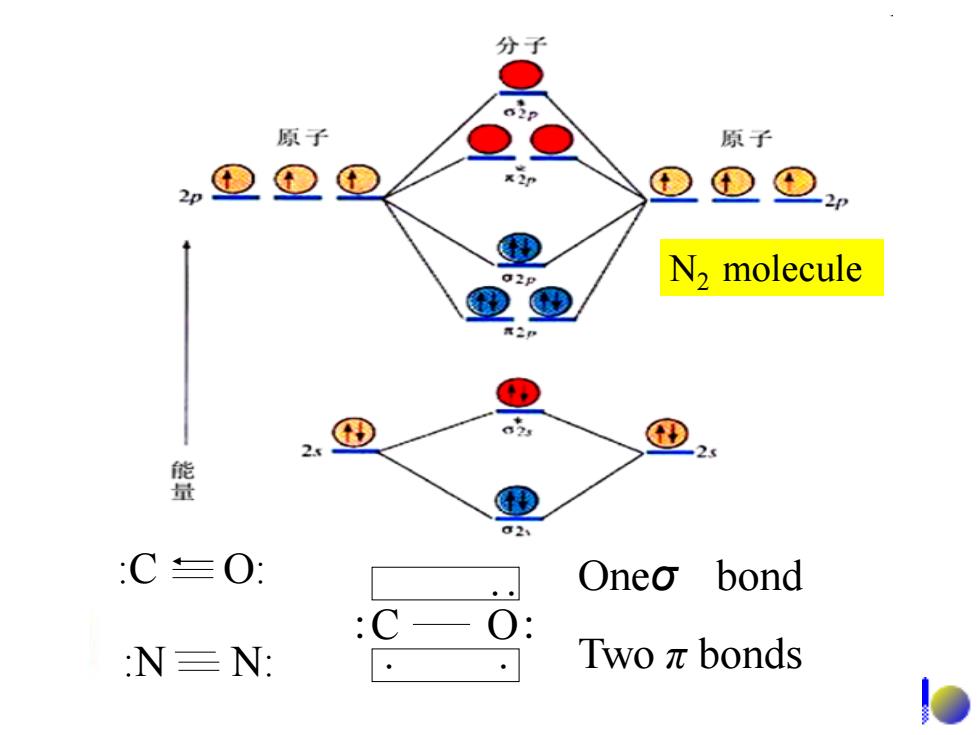
分子 原子 原子 2p ①D =2 02P N2 molecule 2 2 C≡O】 Oneo bond C- :N=N: Twoπbonds
N2 molecule Oneσ bond Two π bonds :C O: :C O: :N N: