正在加载图片...
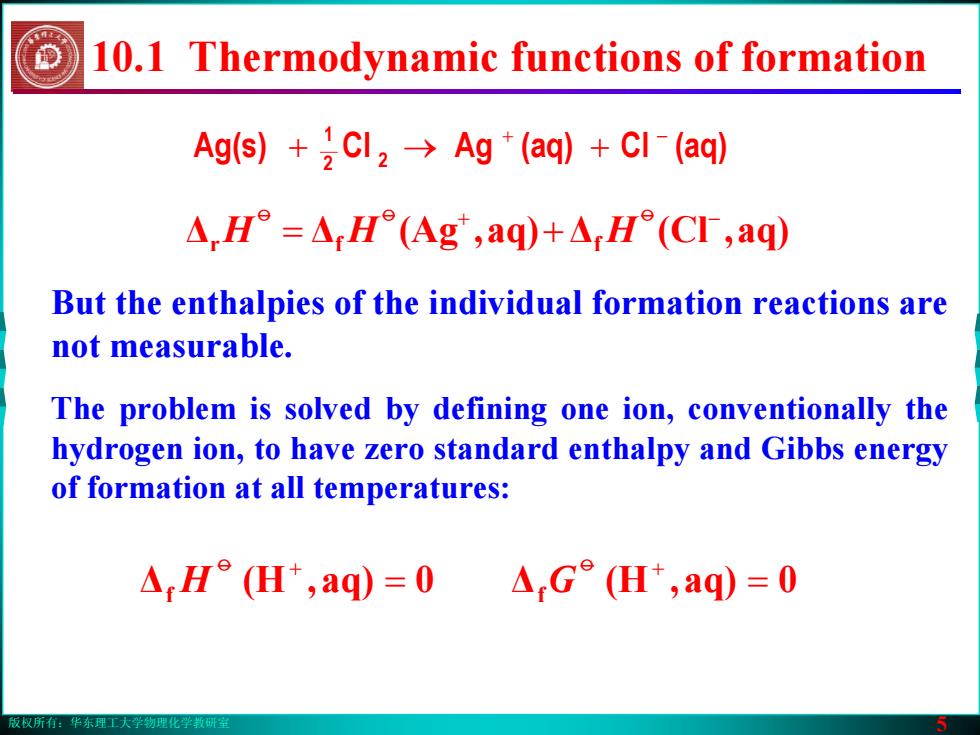
版权所有:华东理工大学物理化学教研室 5 But the enthalpies of the individual formation reactions are not measurable. ΔΔ aq),(Ag Δ aq),(Cl r f f + − = + oo o HH H Ag(s) (aq)Cl(aq)AgCl 2 2 1 + − →+ + The problem is solved by defining one ion, conventionally the hydrogen ion, to have zero standard enthalpy and Gibbs energy of formation at all temperatures: 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H版权所有:华东理工大学物理化学教研室 5 But the enthalpies of the individual formation reactions are not measurable. ΔΔ aq),(Ag Δ aq),(Cl r f f + − = + oo o HH H Ag(s) (aq)Cl(aq)AgCl 2 2 1 + − →+ + The problem is solved by defining one ion, conventionally the hydrogen ion, to have zero standard enthalpy and Gibbs energy of formation at all temperatures: 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H