
版权所有:华东理工大学物理化学教研室 Part 1: Equilibrium 10. Equilibrium electrochemistry
版权所有:华东理工大学物理化学教研室 Part 1: Equilibrium 10. Equilibrium electrochemistry
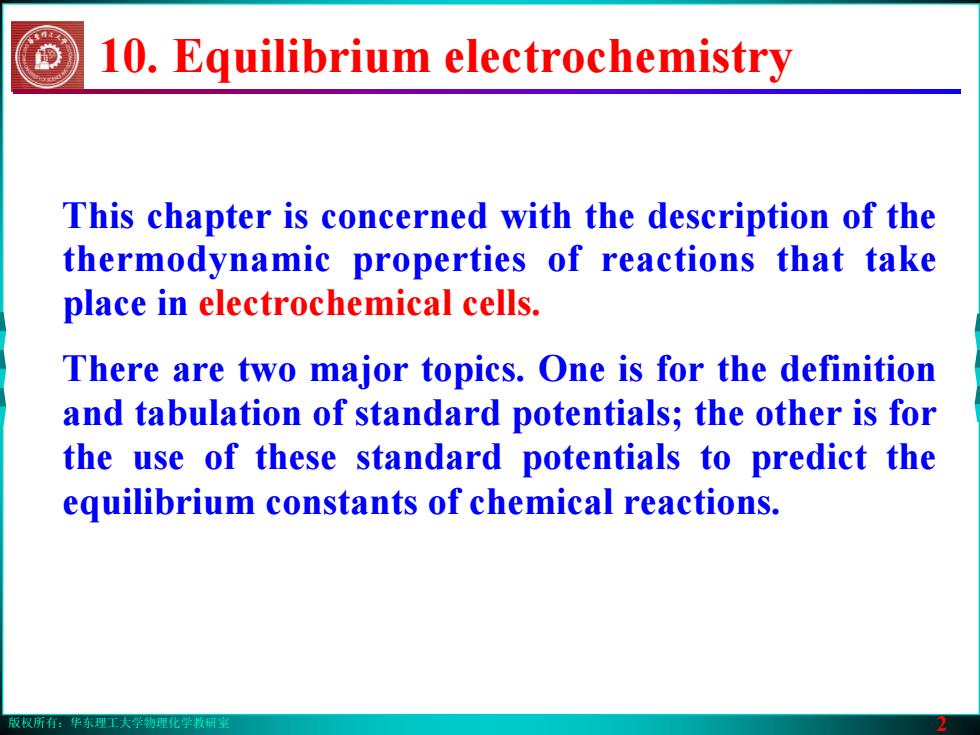
版权所有:华东理工大学物理化学教研室 2 This chapter is concerned with the description of the thermodynamic properties of reactions that take place in electrochemical cells. There are two major topics. One is for the definition and tabulation of standard potentials; the other is for the use of these standard potentials to predict the equilibrium constants of chemical reactions. 10. Equilibrium electrochemistry
版权所有:华东理工大学物理化学教研室 2 This chapter is concerned with the description of the thermodynamic properties of reactions that take place in electrochemical cells. There are two major topics. One is for the definition and tabulation of standard potentials; the other is for the use of these standard potentials to predict the equilibrium constants of chemical reactions. 10. Equilibrium electrochemistry
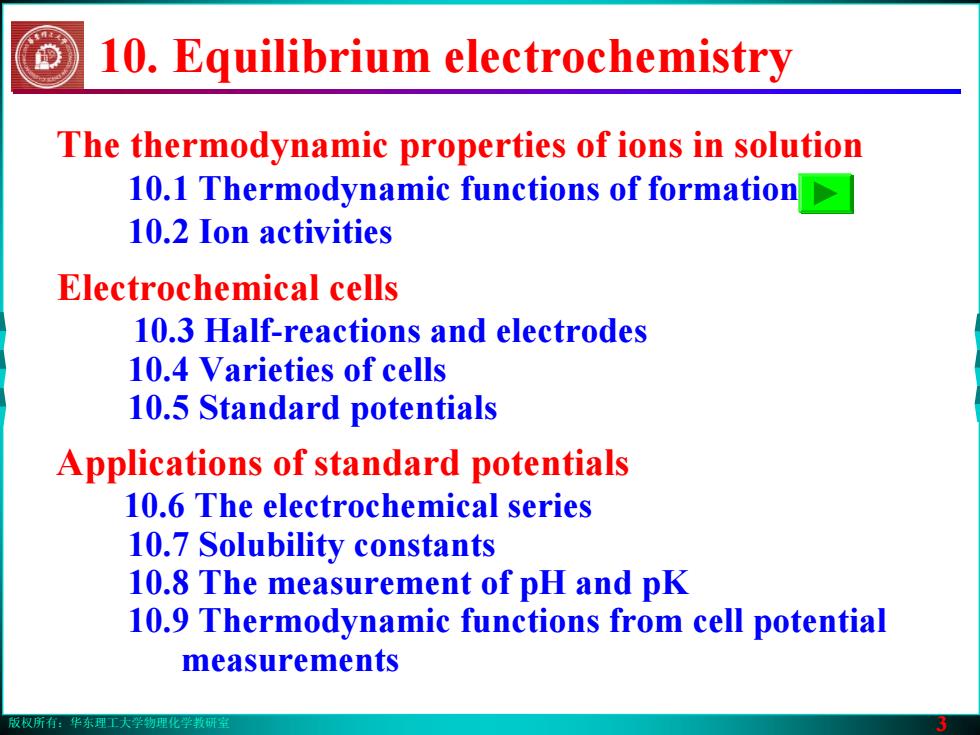
版权所有:华东理工大学物理化学教研室 3 The thermodynamic properties of ions in solution 10.1 Thermodynamic functions of formation 10.2 Ion activities Electrochemical cells 10.3 Half-reactions and electrodes 10.4 Varieties of cells 10.5 Standard potentials Applications of standard potentials 10.6 The electrochemical series 10.7 Solubility constants 10.8 The measurement of pH and pK 10.9 Thermodynamic functions from cell potential measurements 10. Equilibrium electrochemistry
版权所有:华东理工大学物理化学教研室 3 The thermodynamic properties of ions in solution 10.1 Thermodynamic functions of formation 10.2 Ion activities Electrochemical cells 10.3 Half-reactions and electrodes 10.4 Varieties of cells 10.5 Standard potentials Applications of standard potentials 10.6 The electrochemical series 10.7 Solubility constants 10.8 The measurement of pH and pK 10.9 Thermodynamic functions from cell potential measurements 10. Equilibrium electrochemistry

版权所有:华东理工大学物理化学教研室 4 10.1 Thermodynamic functions of formation 1) Standard functions of formation of ions The standard enthalpy and Gibbs energy of a reaction involving ions in solution are expressed in terms of standard enthalpies and Gibbs energies of formation, which can be used in exactly the same way as those for neutral compounds. The values of and refer to the formation of solutions of ions from the reference states of the parent elements. However, solutions of cations cannot be prepared without their accompanying anions. Δf o Δf G o H
版权所有:华东理工大学物理化学教研室 4 10.1 Thermodynamic functions of formation 1) Standard functions of formation of ions The standard enthalpy and Gibbs energy of a reaction involving ions in solution are expressed in terms of standard enthalpies and Gibbs energies of formation, which can be used in exactly the same way as those for neutral compounds. The values of and refer to the formation of solutions of ions from the reference states of the parent elements. However, solutions of cations cannot be prepared without their accompanying anions. Δf o Δf G o H
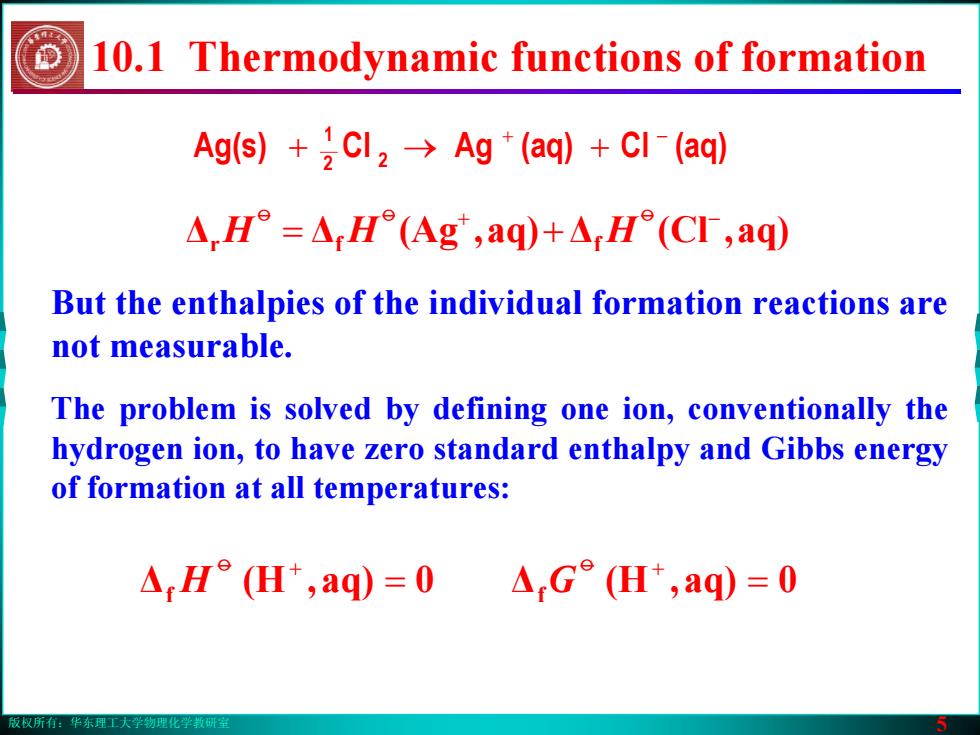
版权所有:华东理工大学物理化学教研室 5 But the enthalpies of the individual formation reactions are not measurable. ΔΔ aq),(Ag Δ aq),(Cl r f f + − = + oo o HH H Ag(s) (aq)Cl(aq)AgCl 2 2 1 + − →+ + The problem is solved by defining one ion, conventionally the hydrogen ion, to have zero standard enthalpy and Gibbs energy of formation at all temperatures: 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H
版权所有:华东理工大学物理化学教研室 5 But the enthalpies of the individual formation reactions are not measurable. ΔΔ aq),(Ag Δ aq),(Cl r f f + − = + oo o HH H Ag(s) (aq)Cl(aq)AgCl 2 2 1 + − →+ + The problem is solved by defining one ion, conventionally the hydrogen ion, to have zero standard enthalpy and Gibbs energy of formation at all temperatures: 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H
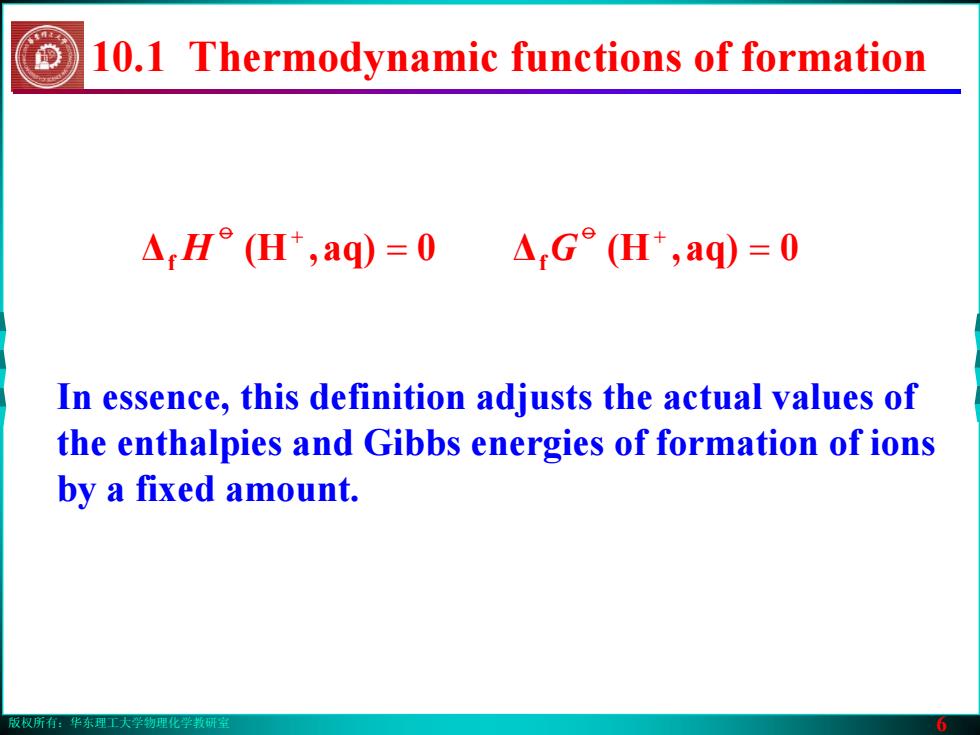
版权所有:华东理工大学物理化学教研室 6 In essence, this definition adjusts the actual values of the enthalpies and Gibbs energies of formation of ions by a fixed amount. 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H
版权所有:华东理工大学物理化学教研室 6 In essence, this definition adjusts the actual values of the enthalpies and Gibbs energies of formation of ions by a fixed amount. 10.1 Thermodynamic functions of formation Δ 0aq),(H f = o + Δf = 0aq),(H G o + H
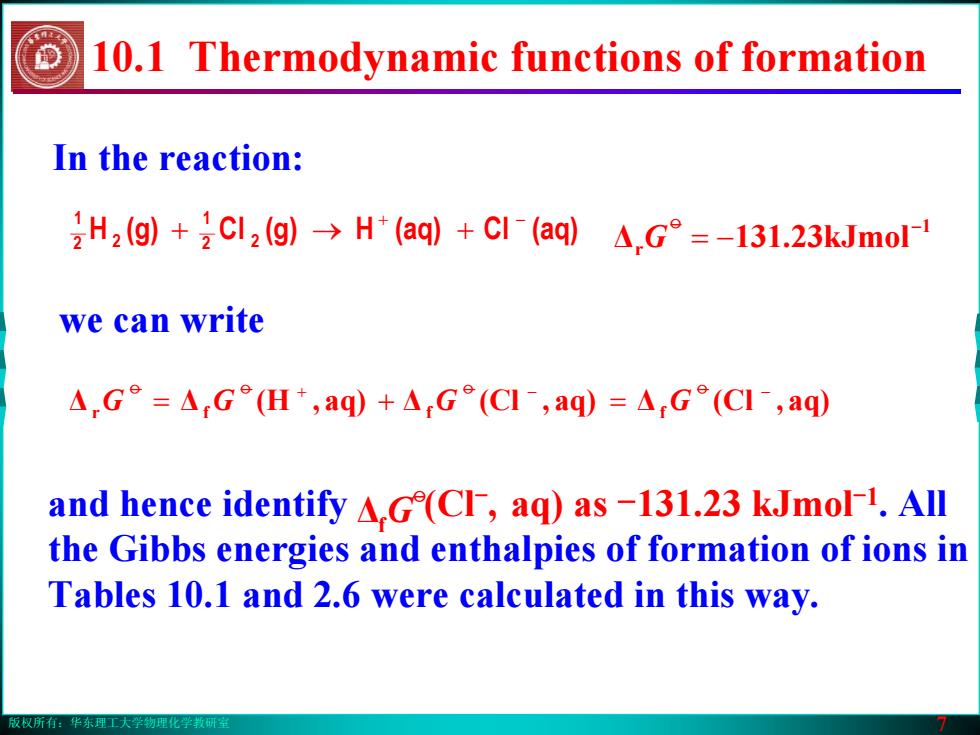
版权所有:华东理工大学物理化学教研室 7 In the reaction: 2 (aq)Cl(aq)H(g)Cl(g)H 21 2 2 1 + − + → + we can write ΔΔ aq),(H Δ aq),(Cl Δ aq),(Cl r f f f + − − = + = oo o o GG G G 1 Δr kJmol23.311 − −= o G and hence identify (Cl-, aq) as -131.23 kJmol-1. All the Gibbs energies and enthalpies of formation of ions in Tables 10.1 and 2.6 were calculated in this way. Δf o G 10.1 Thermodynamic functions of formation
版权所有:华东理工大学物理化学教研室 7 In the reaction: 2 (aq)Cl(aq)H(g)Cl(g)H 21 2 2 1 + − + → + we can write ΔΔ aq),(H Δ aq),(Cl Δ aq),(Cl r f f f + − − = + = oo o o GG G G 1 Δr kJmol23.311 − −= o G and hence identify (Cl-, aq) as -131.23 kJmol-1. All the Gibbs energies and enthalpies of formation of ions in Tables 10.1 and 2.6 were calculated in this way. Δf o G 10.1 Thermodynamic functions of formation
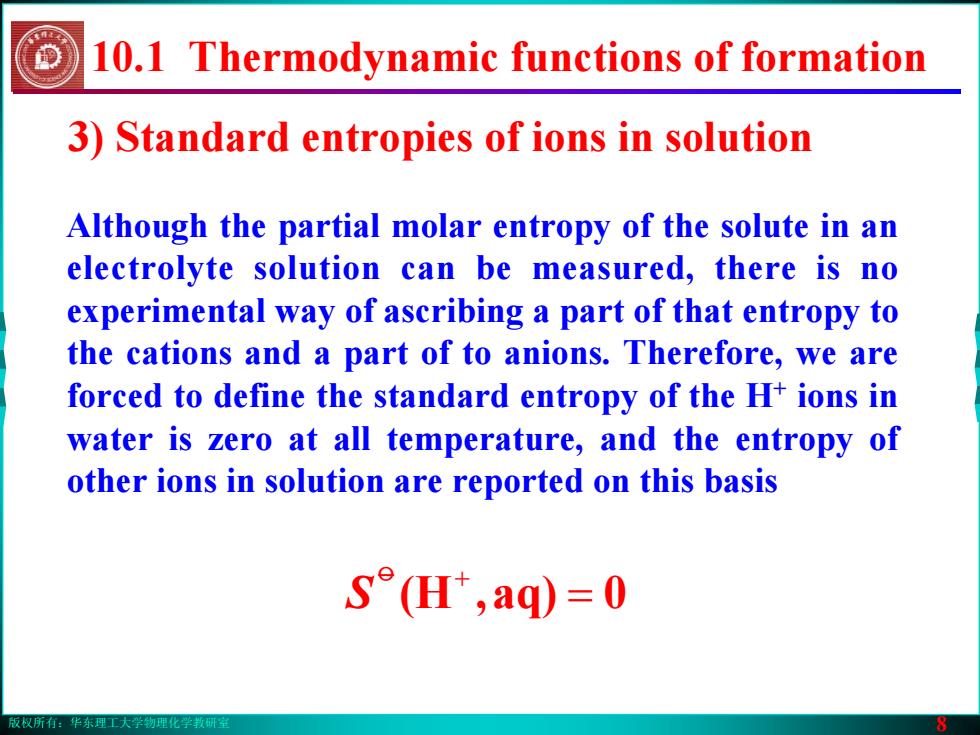
版权所有:华东理工大学物理化学教研室 8 Although the partial molar entropy of the solute in an electrolyte solution can be measured, there is no experimental way of ascribing a part of that entropy to the cations and a part of to anions. Therefore, we are forced to define the standard entropy of the H+ ions in water is zero at all temperature, and the entropy of other ions in solution are reported on this basis 3) Standard entropies of ions in solution = 0aq),(Ho + S 10.1 Thermodynamic functions of formation
版权所有:华东理工大学物理化学教研室 8 Although the partial molar entropy of the solute in an electrolyte solution can be measured, there is no experimental way of ascribing a part of that entropy to the cations and a part of to anions. Therefore, we are forced to define the standard entropy of the H+ ions in water is zero at all temperature, and the entropy of other ions in solution are reported on this basis 3) Standard entropies of ions in solution = 0aq),(Ho + S 10.1 Thermodynamic functions of formation
版权所有:华东理工大学物理化学教研室 9 Because the entropies of ions in water are values relative to the hydrogen ion in water, they may be either positive or negative. 3) Standard entropies of ions in solution A negative entropy means that the ion has a lower partial molar entropy than H+ in water. A positive entropy means that the ion has a higher partial molar entropy than H+ in water 10.1 Thermodynamic functions of formation
版权所有:华东理工大学物理化学教研室 9 Because the entropies of ions in water are values relative to the hydrogen ion in water, they may be either positive or negative. 3) Standard entropies of ions in solution A negative entropy means that the ion has a lower partial molar entropy than H+ in water. A positive entropy means that the ion has a higher partial molar entropy than H+ in water 10.1 Thermodynamic functions of formation

版权所有:华东理工大学物理化学教研室 10 The thermodynamic properties of ions in solution 10.1 Thermodynamic functions of formation 10.2 Ion activities Electrochemical cells 10.3 Half-reactions and electrodes 10.4 Varieties of cells 10.5 Standard potentials Applications of standard potentials 10.6 The electrochemical series 10.7 Solubility constants 10.8 The measurement of pH and pK 10.9 Thermodynamic functions from cell potential measurements 10. Equilibrium electrochemistry
版权所有:华东理工大学物理化学教研室 10 The thermodynamic properties of ions in solution 10.1 Thermodynamic functions of formation 10.2 Ion activities Electrochemical cells 10.3 Half-reactions and electrodes 10.4 Varieties of cells 10.5 Standard potentials Applications of standard potentials 10.6 The electrochemical series 10.7 Solubility constants 10.8 The measurement of pH and pK 10.9 Thermodynamic functions from cell potential measurements 10. Equilibrium electrochemistry