正在加载图片...
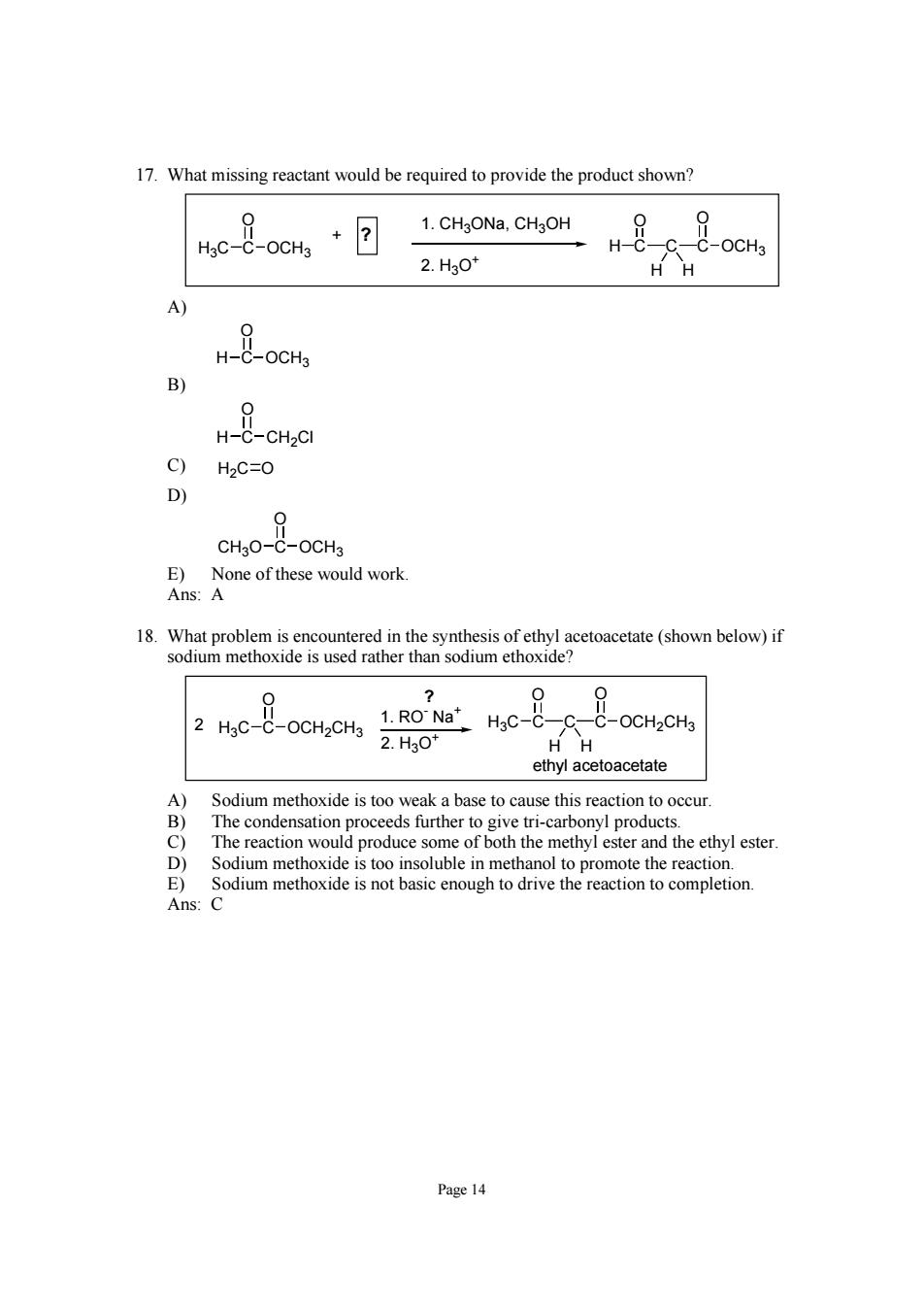
17.What missing reactant would be required to provide the product shown? hc-8oo,+回 1.CHgONa,CHgOH 00 H-C- -C-OCHa 2.H30* H-BOCH B) C)H2C=O D) CHC No of thcse would work 18.What problem is encountered in the synthesis of ethyl acetoacetate(shown below)if sodium methoxide is used rather than sodium ethoxide? 2we-8ocogHc-8t-ocuc 2.H30* HH ethyl acetoacetate A)Sodium methoxide is too weak a base to cause this reaction to occur. The condensation proceeds further to give tri-carbonyl products. 9 The reaction would produce some of both the methyl ester and the ethyl ester. Sodium methoxide is too insoluble in methanol to promote the reaction. Page 14Page 14 17. What missing reactant would be required to provide the product shown? H3C C O OCH3 1. CH3ONa, CH3OH 2. H3O+ + ? C C O C OCH3 O H H H A) H C O OCH3 B) H C O CH2Cl C) H2C O D) CH3O C O OCH3 E) None of these would work. Ans: A 18. What problem is encountered in the synthesis of ethyl acetoacetate (shown below) if sodium methoxide is used rather than sodium ethoxide? C C O C OCH2CH3 O H3C H H H3C C O OCH2CH3 2 1. RO- Na+ 2. H3O+ ethyl acetoacetate ? A) Sodium methoxide is too weak a base to cause this reaction to occur. B) The condensation proceeds further to give tri-carbonyl products. C) The reaction would produce some of both the methyl ester and the ethyl ester. D) Sodium methoxide is too insoluble in methanol to promote the reaction. E) Sodium methoxide is not basic enough to drive the reaction to completion. Ans: C