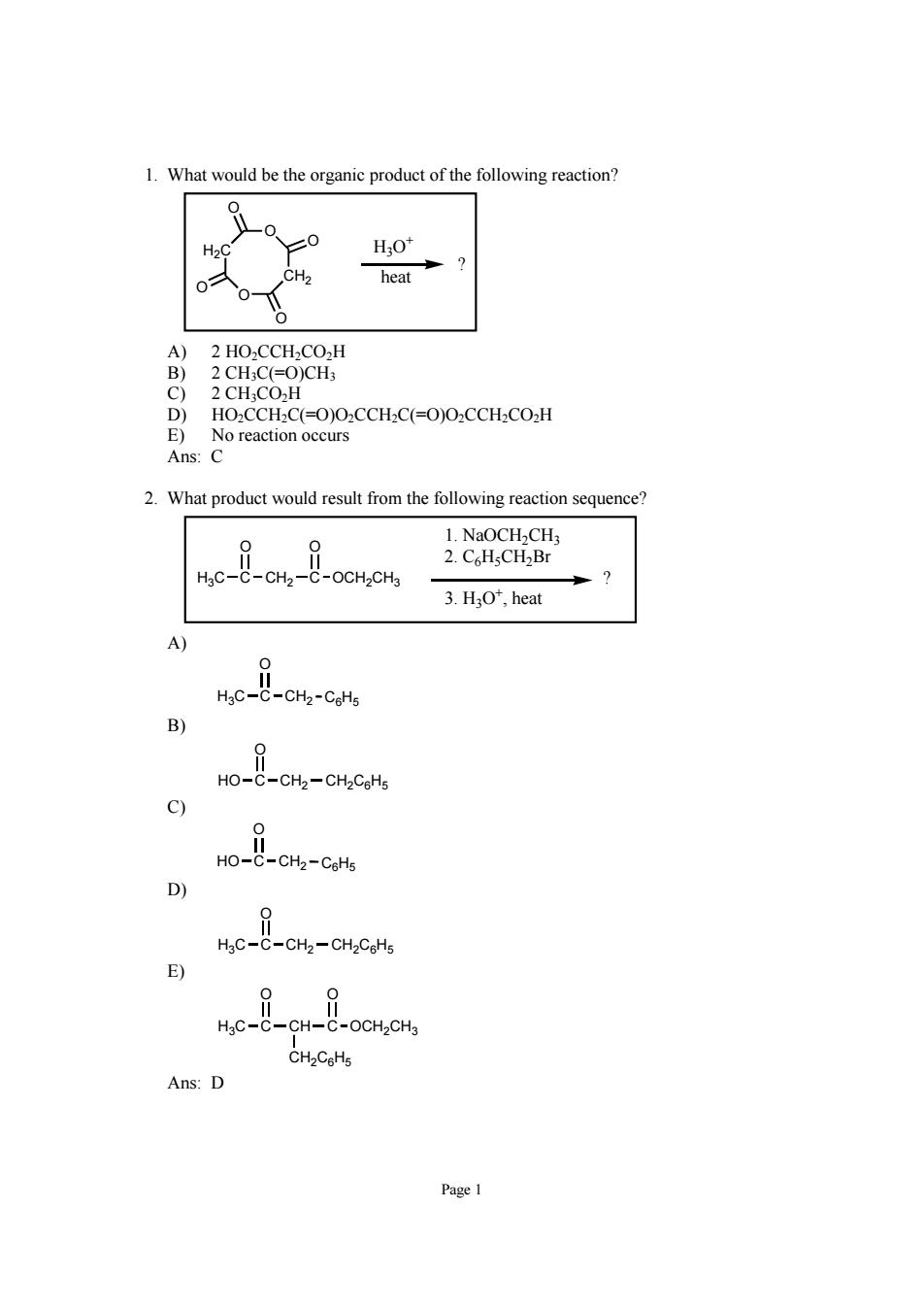
.What would be the organic product of the following reaction H2O* CH2 heat O)CH 2CHCO- HO2CCH2C(=0)02CCH2C(=0)O2CCH2CO2H E)No reaction occurs Ans:C 2.What product would result from the following reaction sequence? 1.NaOCH2CH me-cn 2.C.HsCH2Br -OCH2CH3 3.H:O*,heat we-o HO-C-CH2-CH2CoHa wo-- D HgC-C-CHz-CH2CcHs E) HgC-C-CH- C-OCH2CH3 Ans:D Page 1
Page 1 1. What would be the organic product of the following reaction? H2C O CH2 O O O O O H3O+ heat ? A) 2 HO2CCH2CO2H B) 2 CH3C(=O)CH3 C) 2 CH3CO2H D) HO2CCH2C(=O)O2CCH2C(=O)O2CCH2CO2H E) No reaction occurs Ans: C 2. What product would result from the following reaction sequence? H3C C CH2 C O O OCH2CH3 1. NaOCH2CH3 2. C6H5CH2Br 3. H3O+, heat ? A) H3C C CH2 O C6H5 B) HO C CH2 O CH2C6H5 C) HO C CH2 O C6H5 D) H3C C CH2 O CH2C6H5 E) H3C C CH C O O OCH2CH3 CH2C6H5 Ans: D
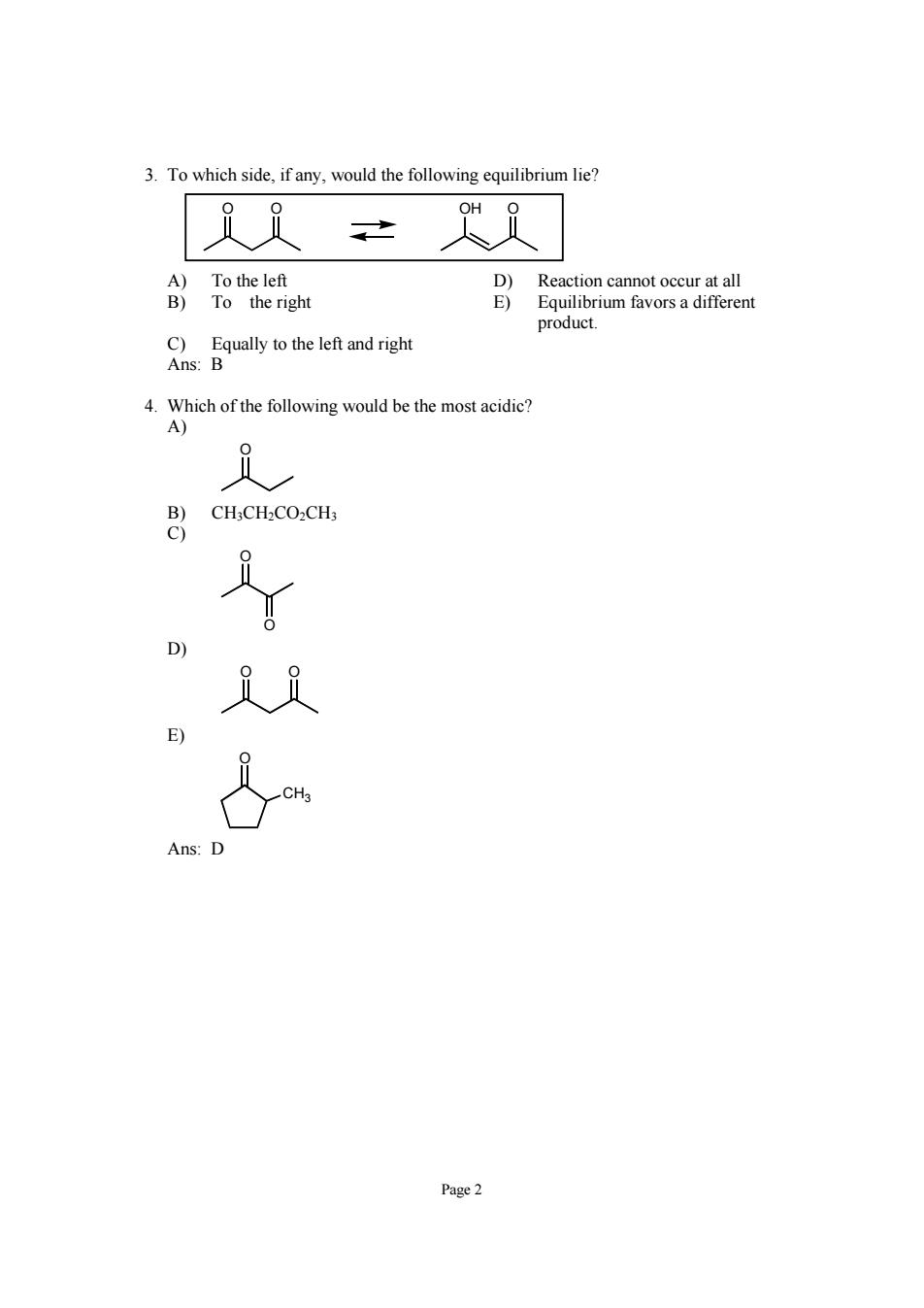
3.To which side.if any,would the following equilibrium lie? 之 )T n D)Reaction cannot occur at all E)Equilibrium favors a different product. C)Equally to the left and right Ans:B .Which ofth followingwoud b the mostacidic CH.CH.CO.CH E) Ans:D Page2
Page 2 3. To which side, if any, would the following equilibrium lie? O O OH O A) To the left D) Reaction cannot occur at all B) To the right E) Equilibrium favors a different product. C) Equally to the left and right Ans: B 4. Which of the following would be the most acidic? A) O B) CH3CH2CO2CH3 C) O O D) O O E) O CH3 Ans: D
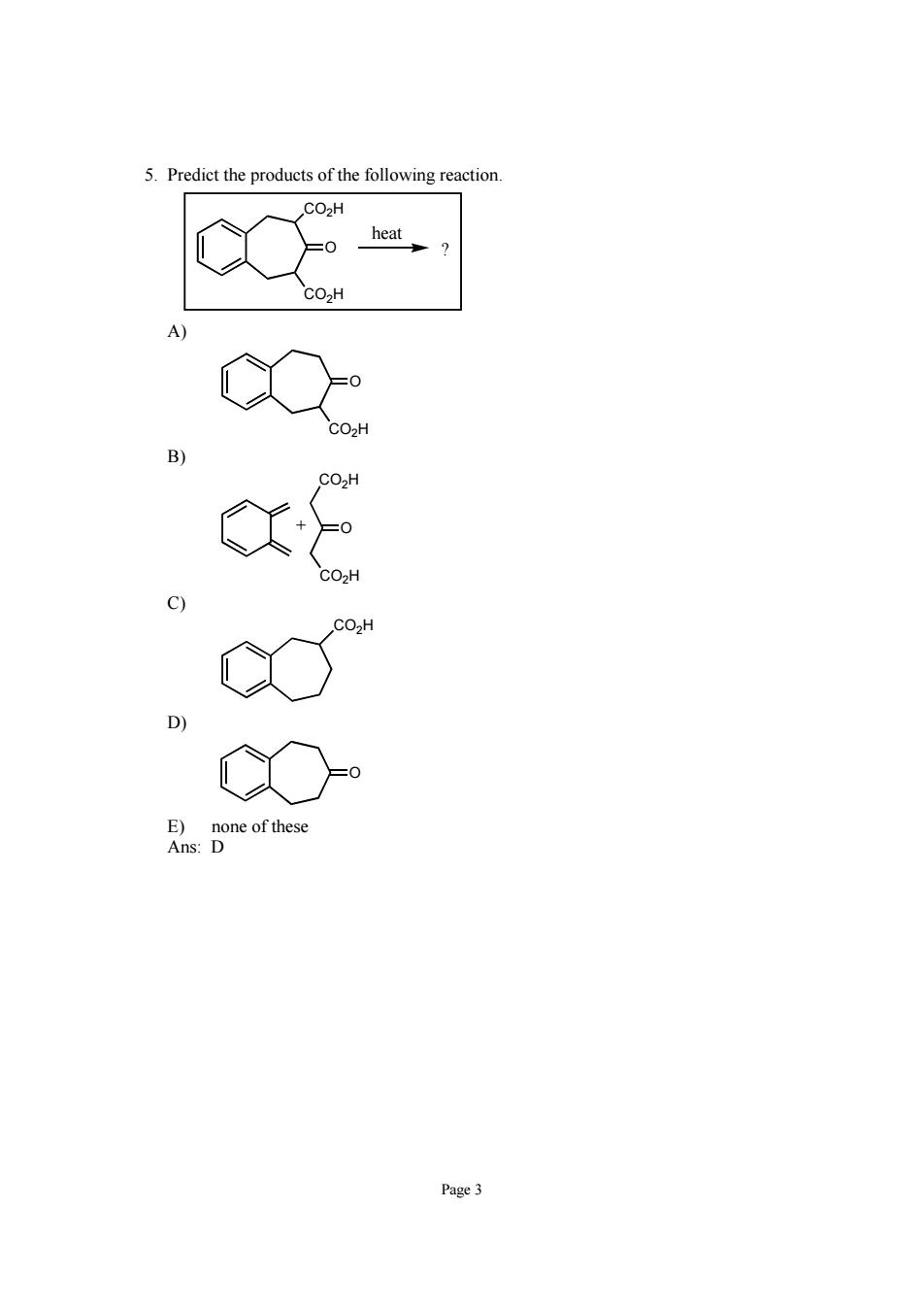
5.Predict the products of the following reactior Co.H Page3
Page 3 5. Predict the products of the following reaction. CO2H CO2H O ? heat A) CO2H O B) CO2H O CO2H + C) CO2H D) O E) none of these Ans: D
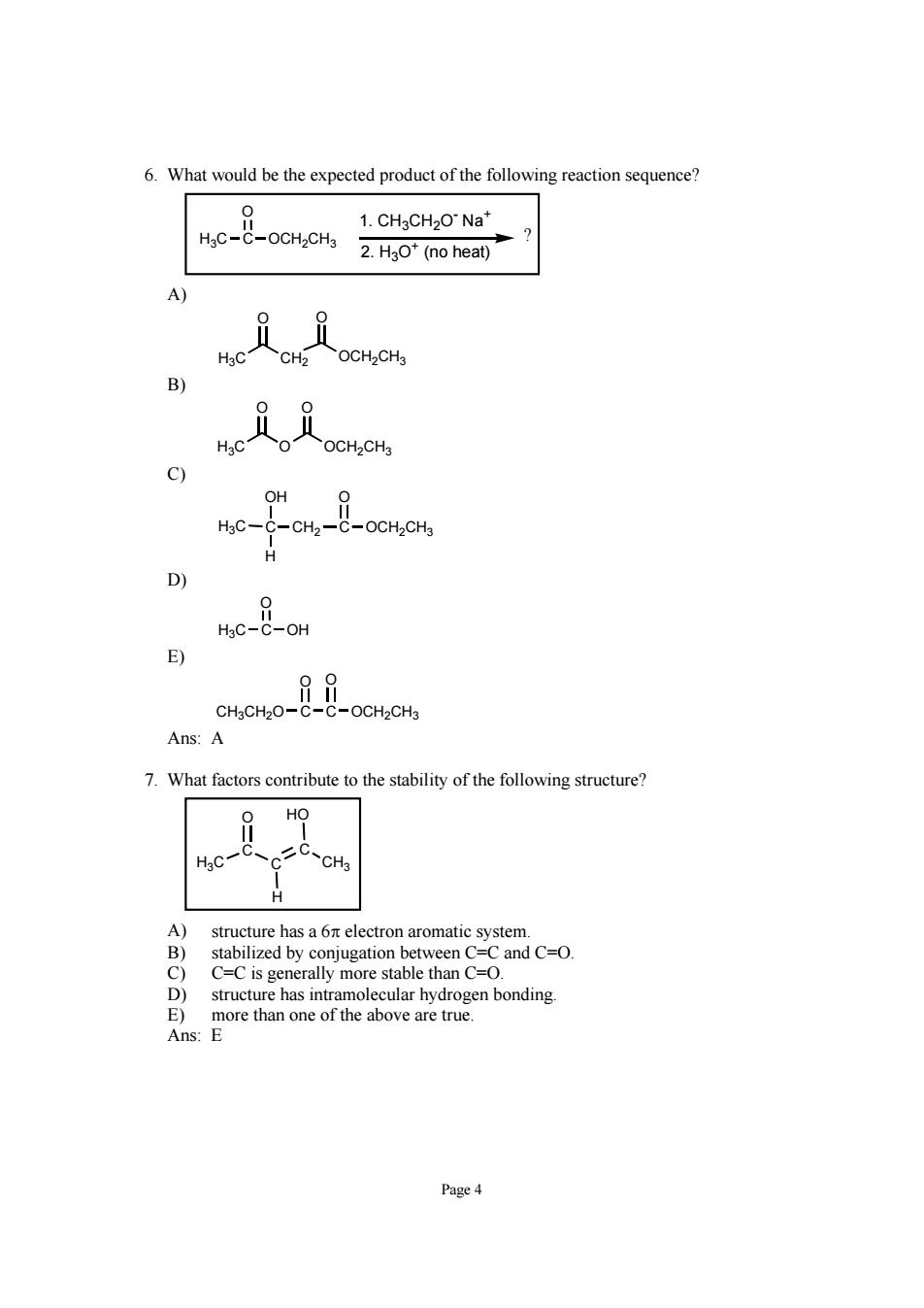
6.What would be the expected product of the following reaction sequence? He8-o0e 1.CHgCH2ONa* 2.HgO*(no heat) 3 H C 9 OH CH,CH wc-o Ans:A 7.What factors contribute to the stability of the following structure? structure has a 6n electron aromatic system. stabilized by conjugation between C-C and C-O C or teren bonding ve are tru Page4
Page 4 6. What would be the expected product of the following reaction sequence? H3C C O OCH2CH3 1. CH3CH2O- Na+ 2. H3O+ (no heat) ? A) H3C CH2 OCH2CH3 O O B) H3C O OCH2CH3 O O C) H3C C CH2 C OCH2CH3 OH O H D) H3C C O OH E) CH3CH2O C O C O OCH2CH3 Ans: A 7. What factors contribute to the stability of the following structure? H3C C C C CH3 O HO H A) structure has a 6π electron aromatic system. B) stabilized by conjugation between C=C and C=O. C) C=C is generally more stable than C=O. D) structure has intramolecular hydrogen bonding. E) more than one of the above are true. Ans: E
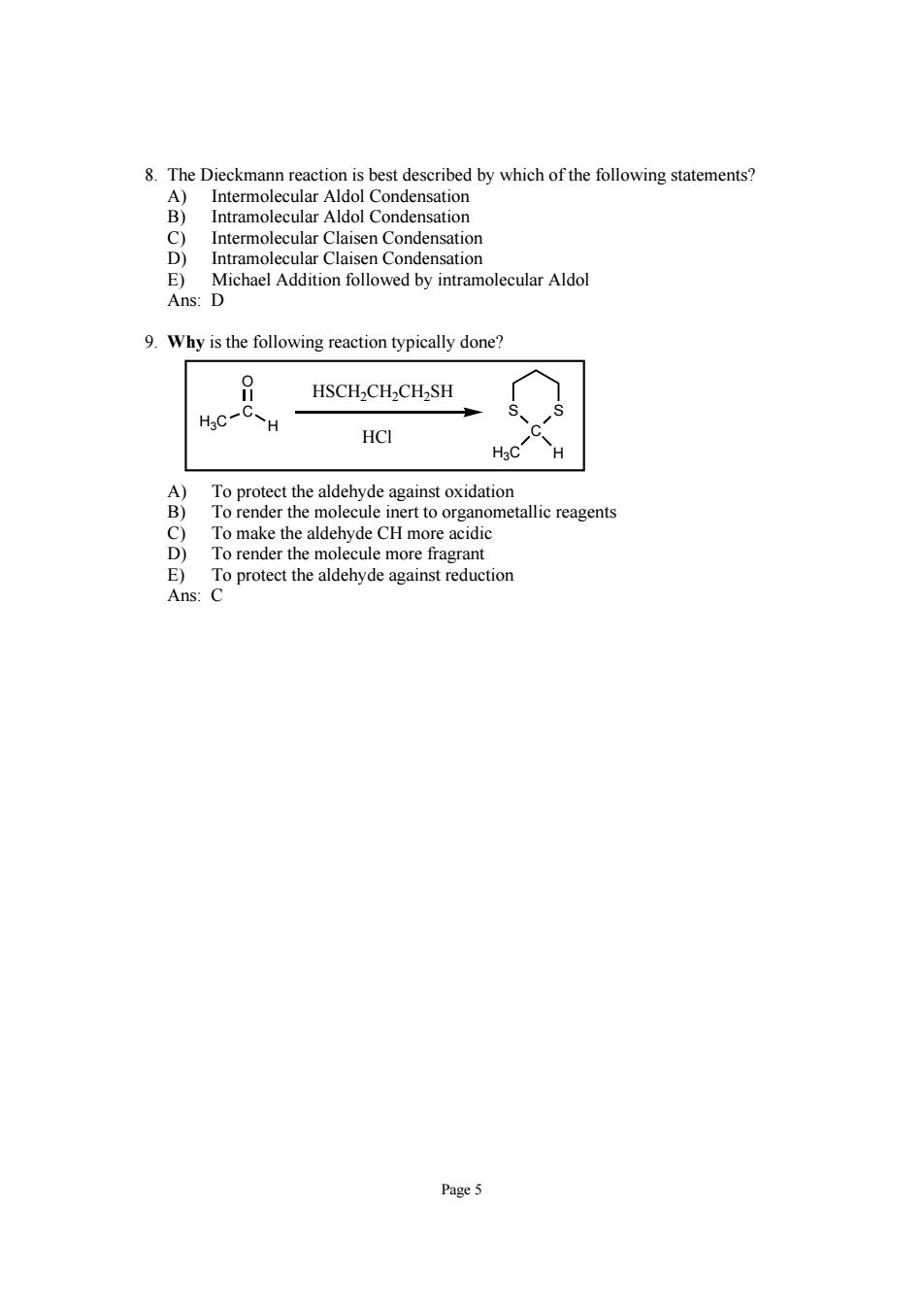
8 Intramolecular Claisen Condensation E)Michael Addition followed by intramolecular Aldol Ans:D 9.Why is the following reaction typically done? HSCH2CH2CH2SH H 3C }8mtckeoetgansoihio To render the molecule inert to organometallic reagents To make the aldehyde CH more acidic To render the molecule more fragrant rohe aeydeuco Page5
Page 5 8. The Dieckmann reaction is best described by which of the following statements? A) Intermolecular Aldol Condensation B) Intramolecular Aldol Condensation C) Intermolecular Claisen Condensation D) Intramolecular Claisen Condensation E) Michael Addition followed by intramolecular Aldol Ans: D 9. Why is the following reaction typically done? S C S H3C H C H3C H O HSCH2CH2CH2SH HCl A) To protect the aldehyde against oxidation B) To render the molecule inert to organometallic reagents C) To make the aldehyde CH more acidic D) To render the molecule more fragrant E) To protect the aldehyde against reduction Ans: C
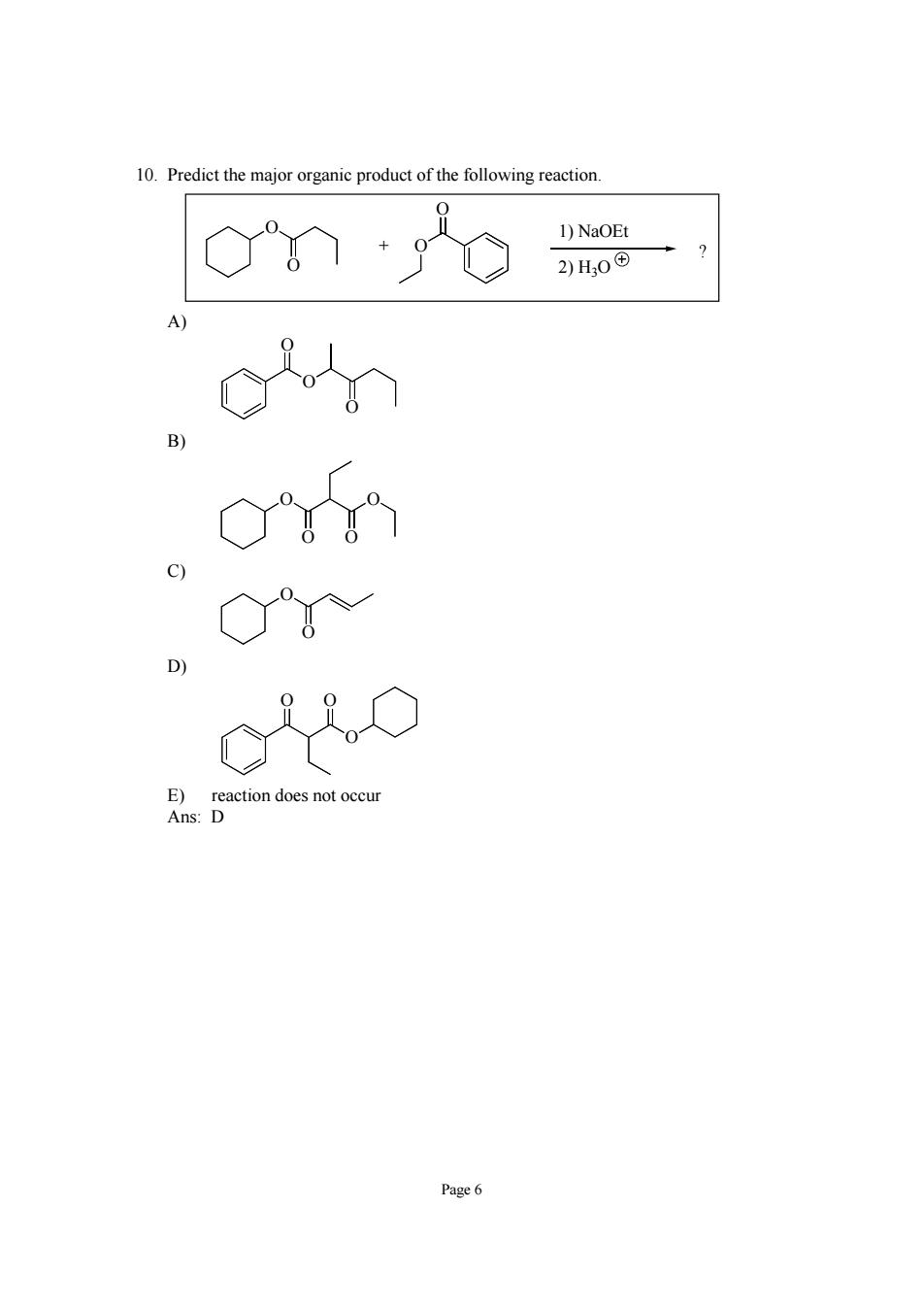
10.Predict the major organic product of the following reaction. 1)NaOEt 2)H,0田 c am 。Y Cio Page6
Page 6 10. Predict the major organic product of the following reaction. O O + O O 1) NaOEt 2) H3O ? A) O O O B) O O O O C) O O D) O O O E) reaction does not occur Ans: D

11.What product results from treatment of the 1.2-diketone below with hydroxide? KOH,H2 100C Page7
Page 7 11. What product results from treatment of the 1,2-diketone below with hydroxide? A) O- Na+ OH O B) Na+ - O O OH C) HO O OH D) HO O E) OH OH Ans: A

12.What product would be expected to result from the reaction sequence shown? 3.NaOH.H2O,heat 4.H3O,heat CH2CH2CH2CH 8 H.CH2CO CH2CH2CH2CH3 HO OCH CH CH2CH2CH2CH HO E)HO2C-CH2CH2CH2CH2CH3 Ans:E Page8
Page 8 12. What product would be expected to result from the reaction sequence shown? H3CH2CO OCH2CH3 O O 1. NaOCH2CH3 2. CH3CH2CH2CH2Br 3. NaOH, H2O, heat 4. H3O+ , heat ? A) H3CH2CO CH2CH2CH2CH3 O O B) H3CH2CO OCH2CH3 O O CH2CH2CH2CH3 C) HO OCH2CH3 O O CH2CH2CH2CH3 D) HO OH O O CH2CH2CH2CH3 E) HO2C-CH2CH2CH2CH2CH3 Ans: E
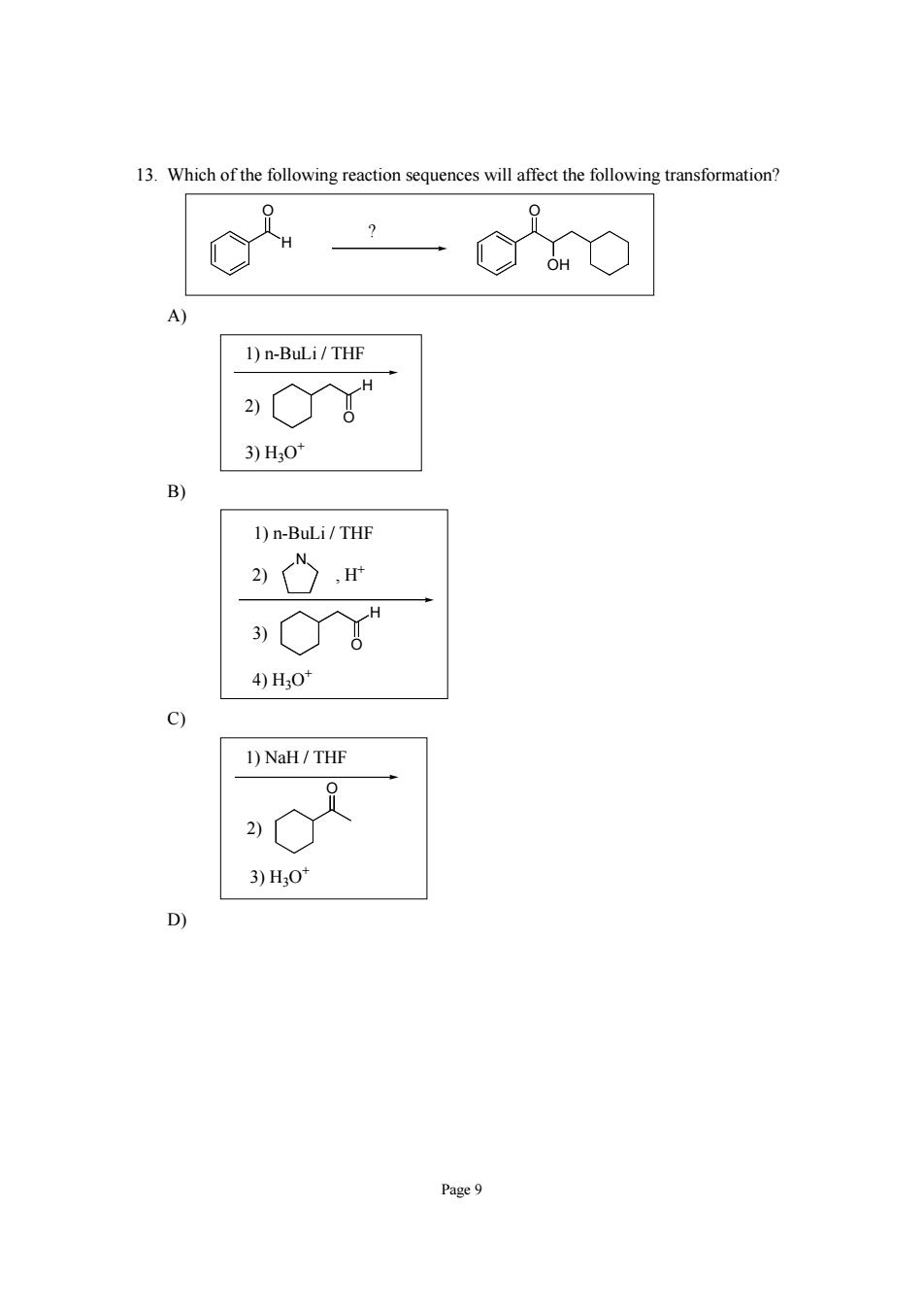
13.Which of the following reaction sequences will affect the following transformation c20 1)n-BuLi/THF 。 3)H,0 B) 1)n-BuLi/THF 心r 。 4)H0 9 1)NaH/THF 3)H,0 D) Page9
Page 9 13. Which of the following reaction sequences will affect the following transformation? H O ? O OH A) 1) n-BuLi / THF 3) H3O+ O H 2) B) 1) n-BuLi / THF 4) H3O+ O H 3) 2) N , H+ C) 1) NaH / THF 3) H3O+ 2) O D)

1)HSCH2CH2CH2SH /ZnClz 2)n-BuLi/THF 4H0 5)HgCl2/H2O/CaCO;/CH3CN 2)H0 Ans:D Page 10
Page 10 2) n-BuLi / THF 1) HSCH2CH2CH2SH /ZnCl2 O H 3) 4) H3O+ 5) HgCl2 / H2O / CaCO3 / CH3CN E) P 1) 2) H3O+ Ans: D