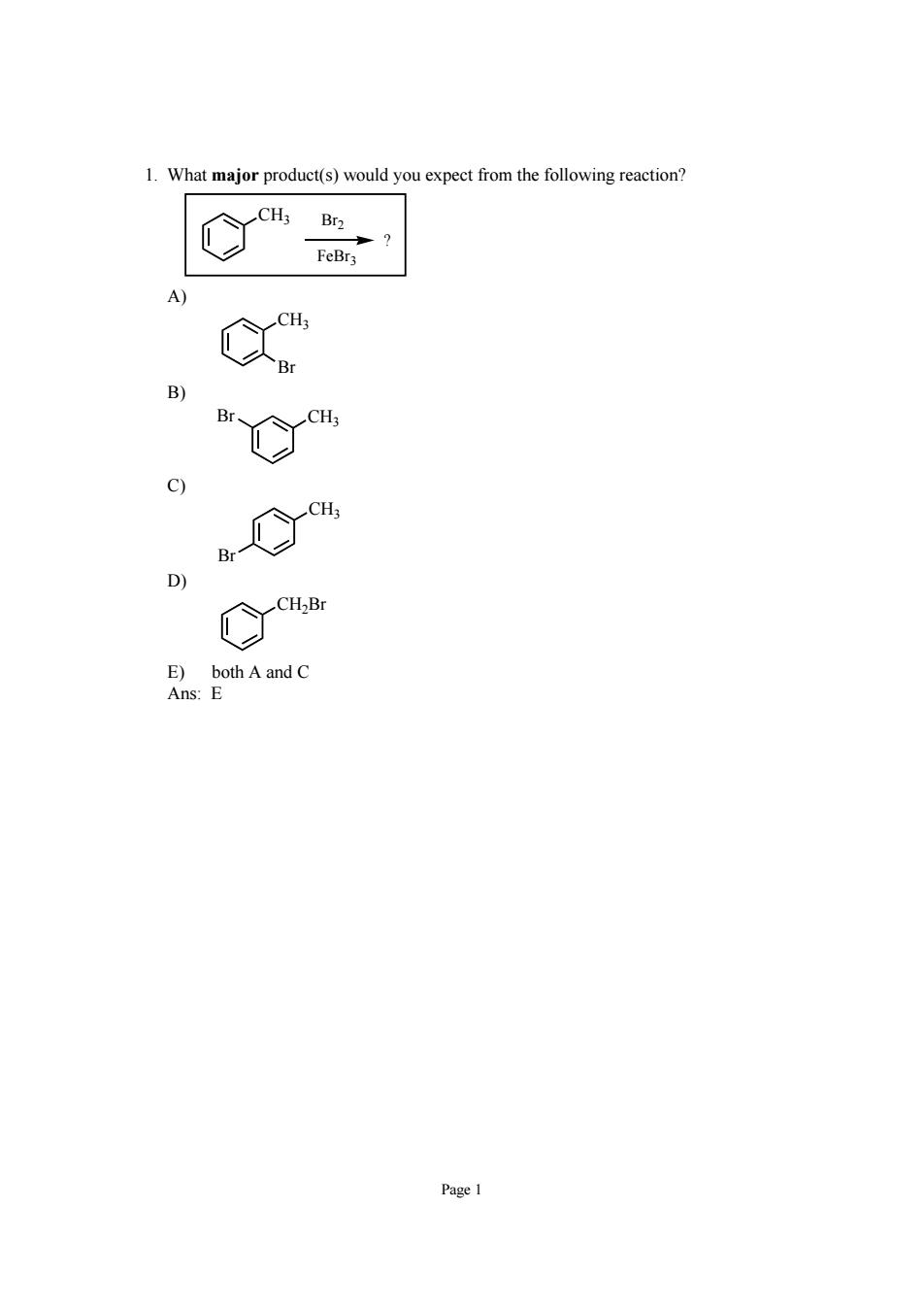
1.What major product(s)would you expect from the following reaction? Br D) ng both A and C Page 1
Page 1 1. What major product(s) would you expect from the following reaction? CH3 ? Br2 FeBr3 A) CH3 Br B) Br CH3 C) CH3 Br D) CH2Br E) both A and C Ans: E
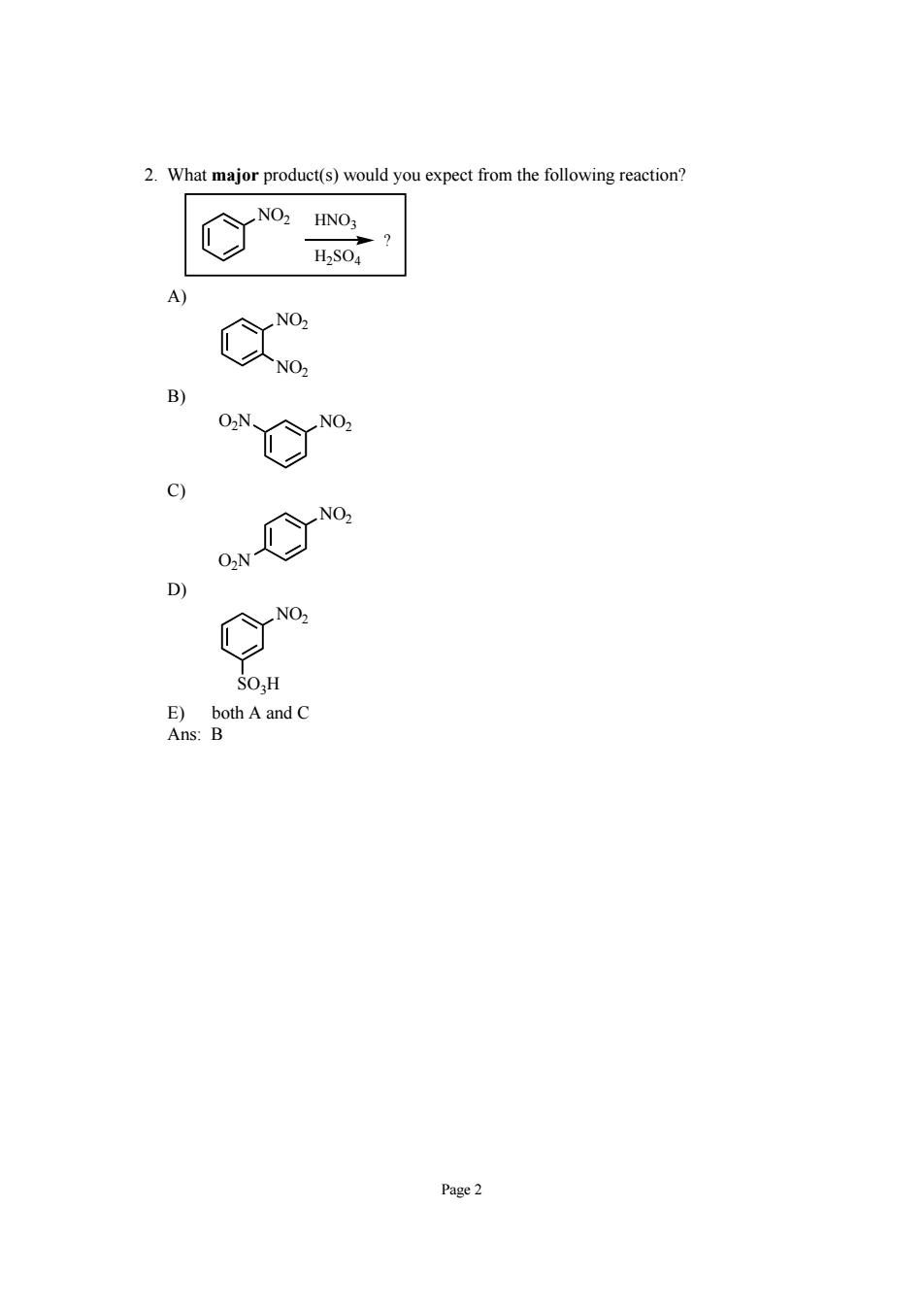
2.What major product(s)would you expect from the following reaction? % C) D No. ns uh A and C Page2
Page 2 2. What major product(s) would you expect from the following reaction? NO2 ? HNO3 H2SO4 A) NO2 NO2 B) O2N NO2 C) NO2 O2N D) NO2 SO3H E) both A and C Ans: B
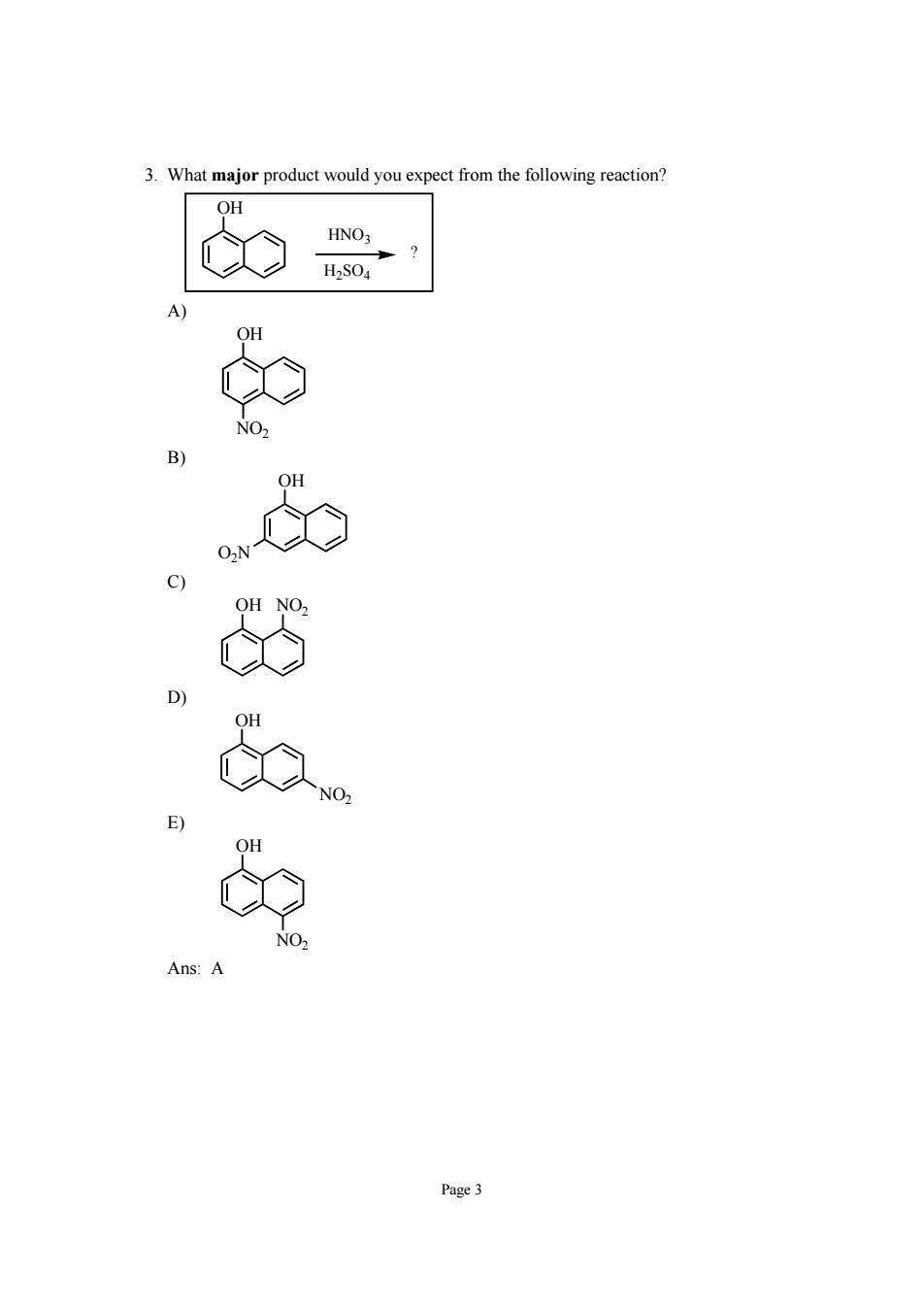
3.What major product would you expect from the following reaction? OH e Page3
Page 3 3. What major product would you expect from the following reaction? OH ? HNO3 H2SO4 A) OH NO2 B) OH O2N C) OH NO2 D) OH NO2 E) OH NO2 Ans: A
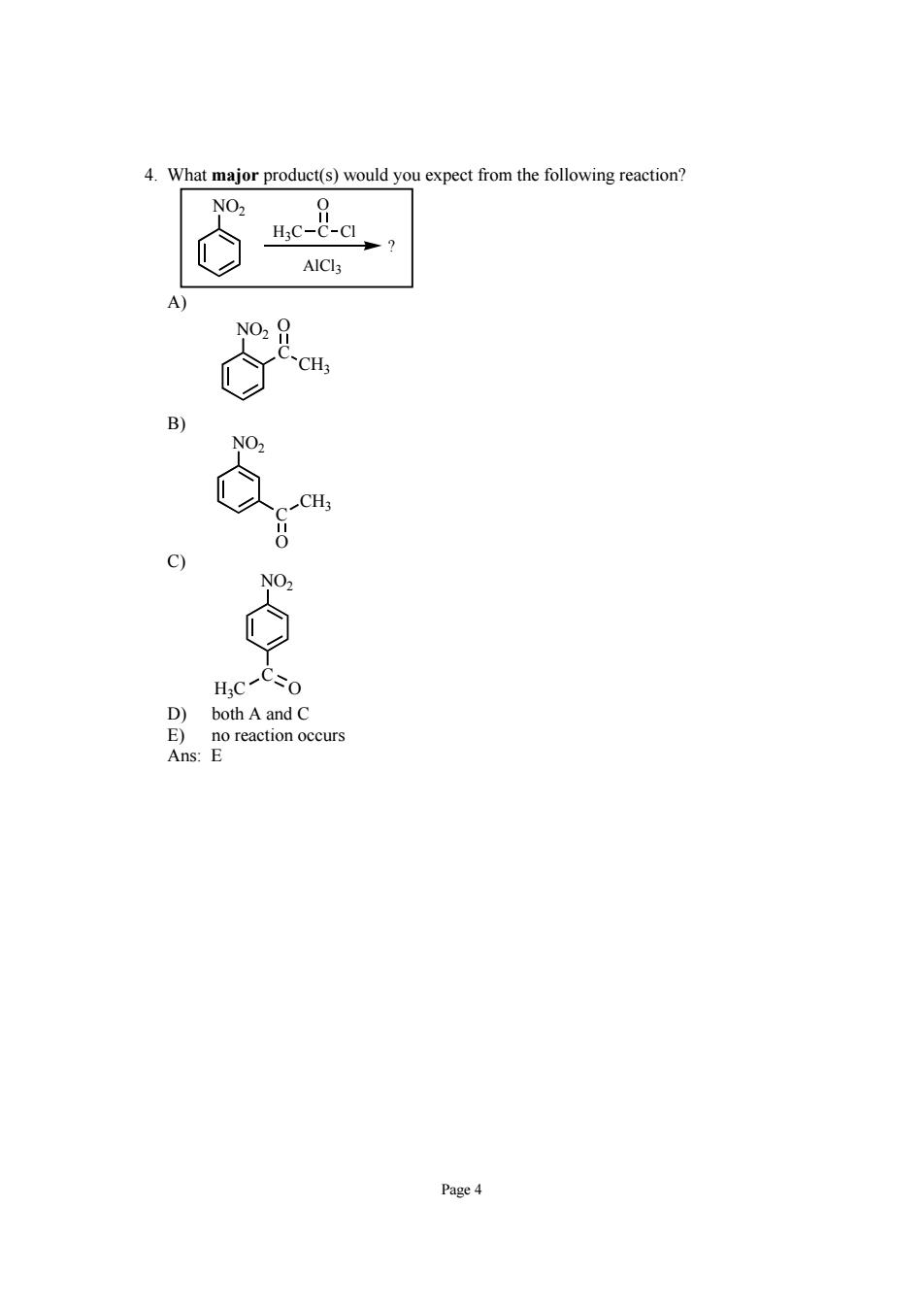
What major product(s)would you expect from the following reaction? net-a AlCla CH 、CH HC-Co D)both A and C E)no reaction occurs Ans:E Page 4
Page 4 4. What major product(s) would you expect from the following reaction? H3C C O Cl NO2 ? AlCl3 A) NO2 C O CH3 B) NO2 O CH3 C C) NO2 H3C O C D) both A and C E) no reaction occurs Ans: E
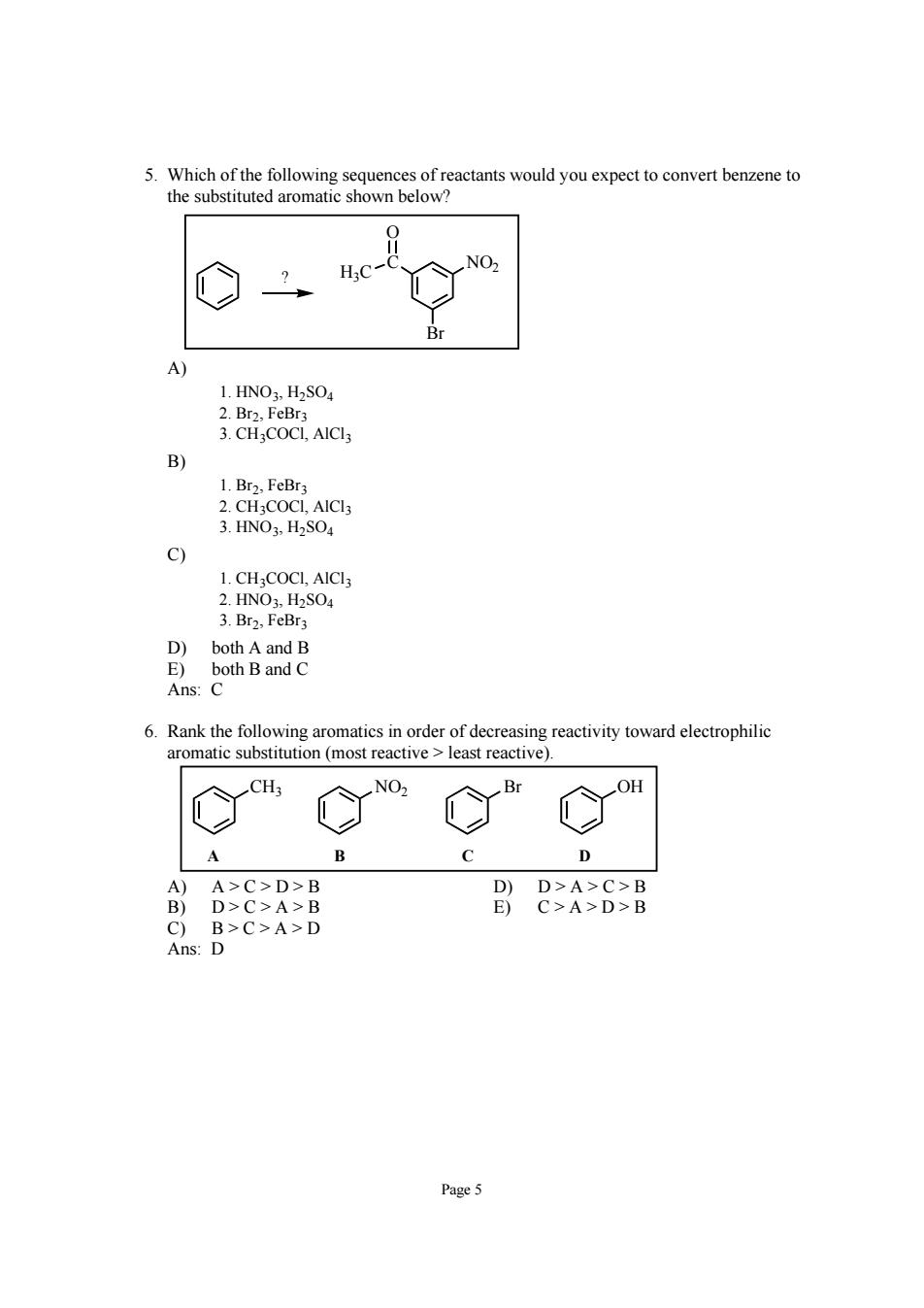
H.C NO 1.HNO3.H2SOa 2.Br2.FeBr3 3.CHCOCL,AICIs C) 1.CHgCOCL,AICI3 2.HN0,H2S04 3.Br2,FeBr D)both A and B E)both Band C Ans:C 6.Rank the following aromatics in order of decreasing reactivity toward electrophilic aromatic substitution (most reactive least reactive). A B C D A>C>D>B D-C>A-D Page5
Page 5 5. Which of the following sequences of reactants would you expect to convert benzene to the substituted aromatic shown below? H C NO2 3C O Br ? A) 1. HNO3, H2SO4 2. Br2, FeBr3 3. CH3COCl, AlCl3 B) 1. Br2, FeBr3 2. CH3COCl, AlCl3 3. HNO3, H2SO4 C) 1. CH3COCl, AlCl3 2. HNO3, H2SO4 3. Br2, FeBr3 D) both A and B E) both B and C Ans: C 6. Rank the following aromatics in order of decreasing reactivity toward electrophilic aromatic substitution (most reactive > least reactive). CH3 NO2 Br OH A B C D A) A > C > D > B D) D > A > C > B B) D > C > A > B E) C > A > D > B C) B > C > A > D Ans: D
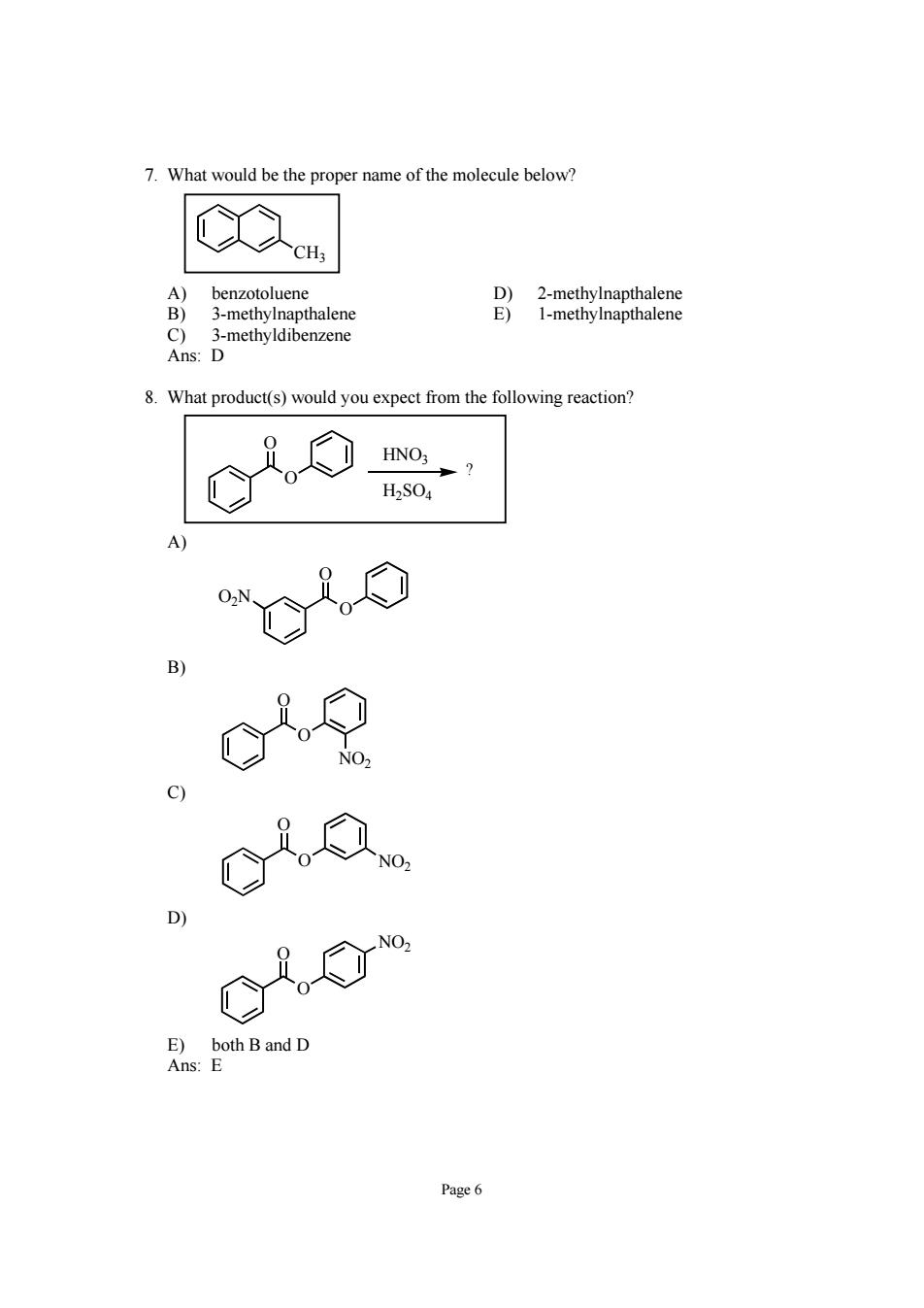
7.What would be the proper name of the molecule below? henzotoluene 3-methylnapthalene 3-methyldibenzene Ans:D 8.What product(s)would you expect from the following reaction? HS04 c29 c) Q C,⑦ E)both Band D Ans:E Page6
Page 6 7. What would be the proper name of the molecule below? CH3 A) benzotoluene D) 2-methylnapthalene B) 3-methylnapthalene E) 1-methylnapthalene C) 3-methyldibenzene Ans: D 8. What product(s) would you expect from the following reaction? O O HNO3 H2SO4 ? A) O O O2N B) O O NO2 C) O O NO2 D) O O NO2 E) both B and D Ans: E
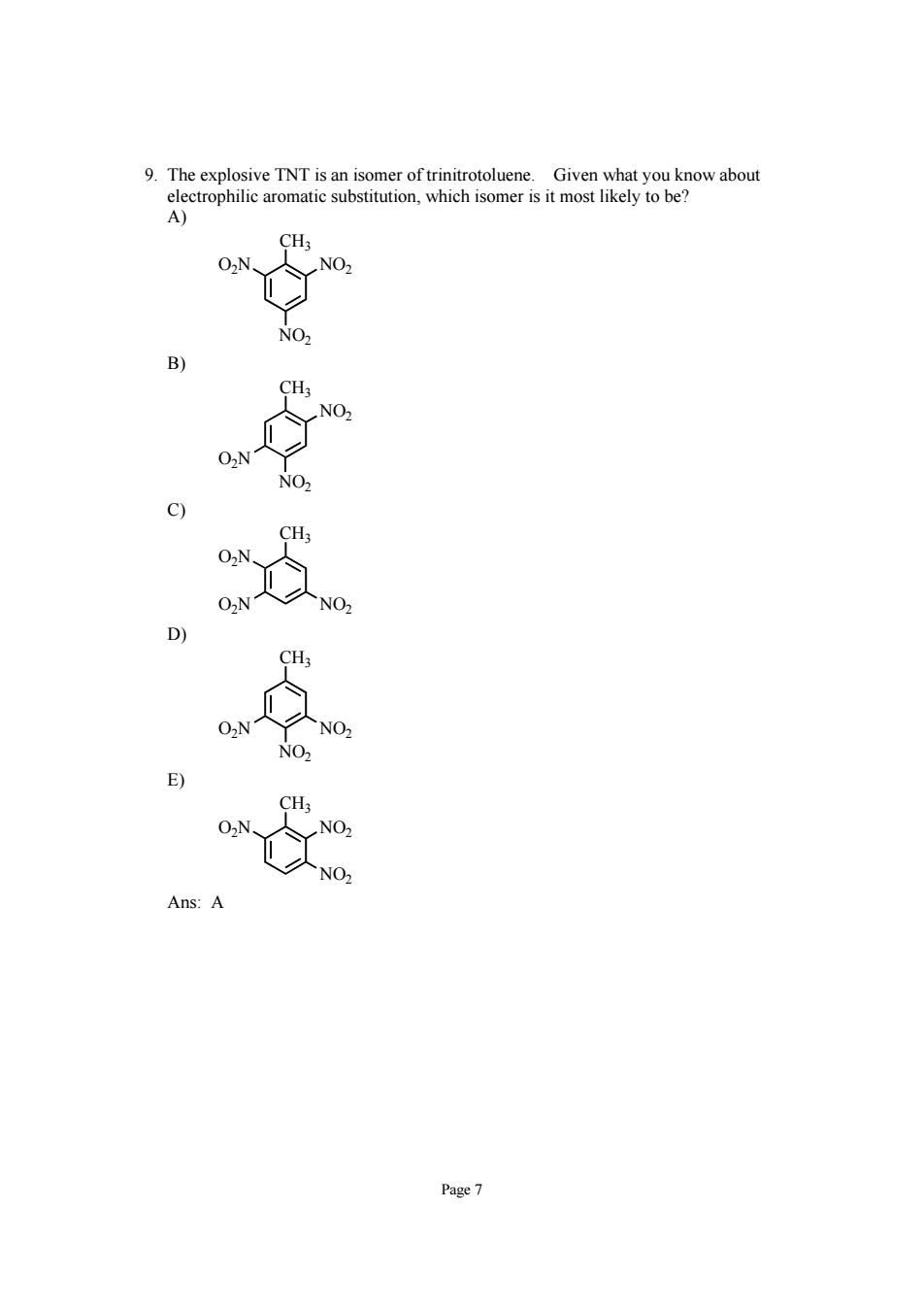
9.The explosive TNT is an isomer oftrinitrotoluene. CH; NO Page7
Page 7 9. The explosive TNT is an isomer of trinitrotoluene. Given what you know about electrophilic aromatic substitution, which isomer is it most likely to be? A) CH3 O2N NO2 NO2 B) CH3 NO2 O2N NO2 C) CH3 O2N O2N NO2 D) CH3 O2N NO2 NO2 E) CH3 O2N NO2 NO2 Ans: A
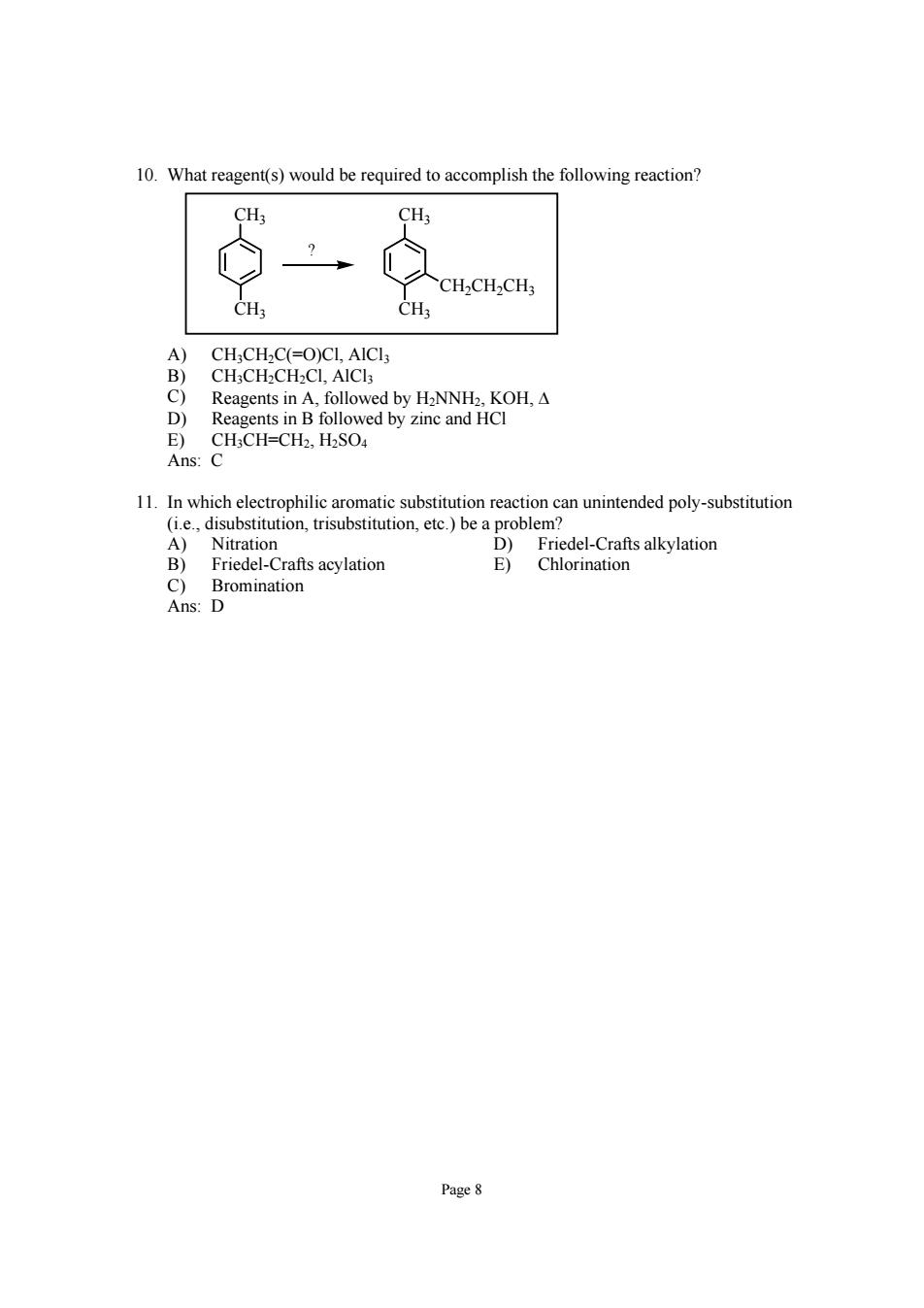
10.What reagent(s)would be required to accomplish the following reaction? CH CHCHCH: CH. CH: CH;CH2C(=O)CI,AICI CH;CH2CH2CI,AICls Reagents in A,followed by HaNNH2.KOH,A followed by zinc and HCI 1laetahsenccoaeeamwhcmcantmnmndipot-atna A)Nitration D)Friedel-Crafts alkylation B)Friedel-Crafts acylation Bromination E)Chlorination Page8
Page 8 10. What reagent(s) would be required to accomplish the following reaction? CH3 CH3 CH3 CH3 CH2CH2CH3 ? A) CH3CH2C(=O)Cl, AlCl3 B) CH3CH2CH2Cl, AlCl3 C) Reagents in A, followed by H2NNH2, KOH, Δ D) Reagents in B followed by zinc and HCl E) CH3CH=CH2, H2SO4 Ans: C 11. In which electrophilic aromatic substitution reaction can unintended poly-substitution (i.e., disubstitution, trisubstitution, etc.) be a problem? A) Nitration D) Friedel-Crafts alkylation B) Friedel-Crafts acylation E) Chlorination C) Bromination Ans: D
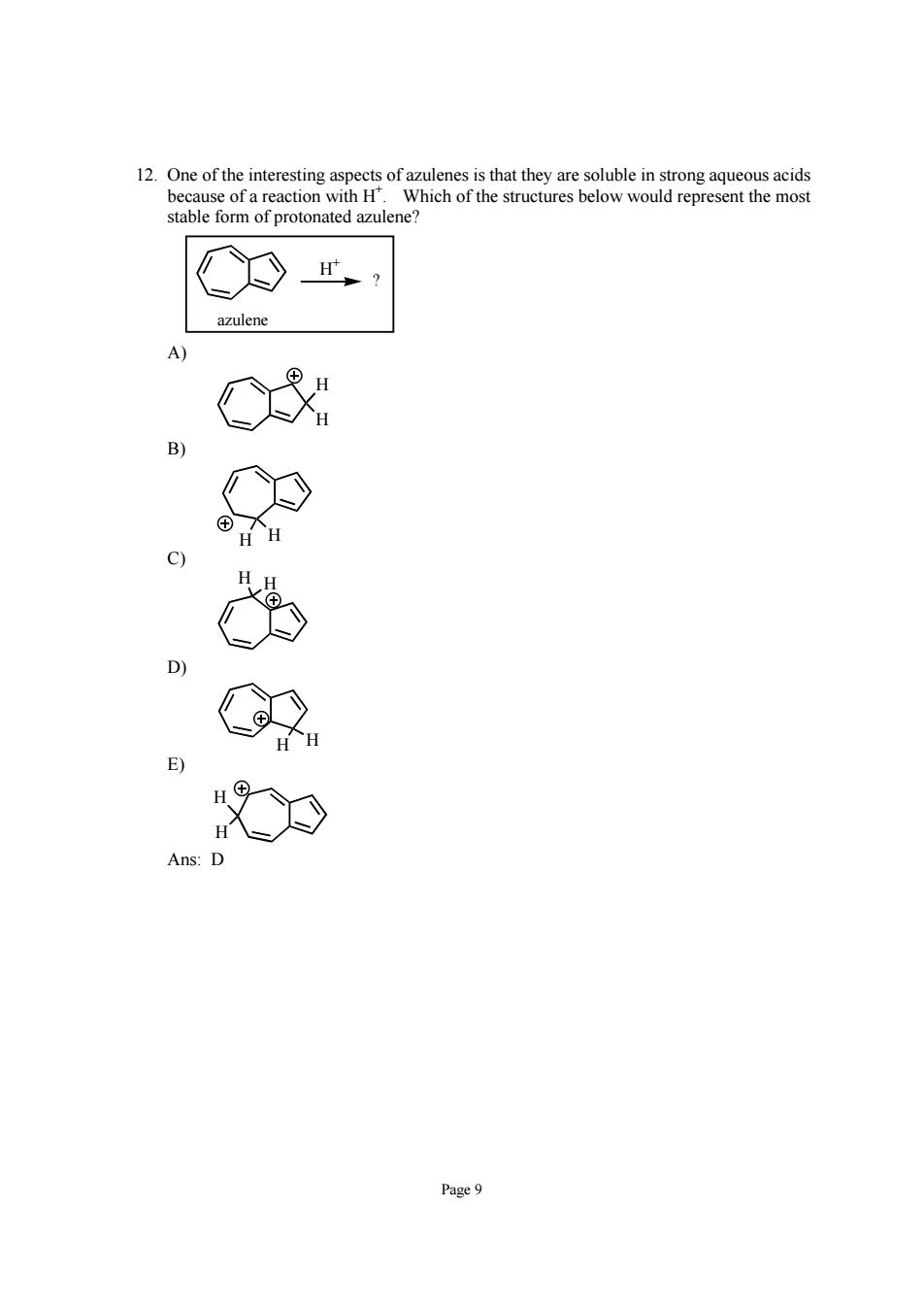
represent the mos Page9
Page 9 12. One of the interesting aspects of azulenes is that they are soluble in strong aqueous acids because of a reaction with H+ . Which of the structures below would represent the most stable form of protonated azulene? ? H+ azulene A) H H B) H H C) H H D) H H E) H H Ans: D
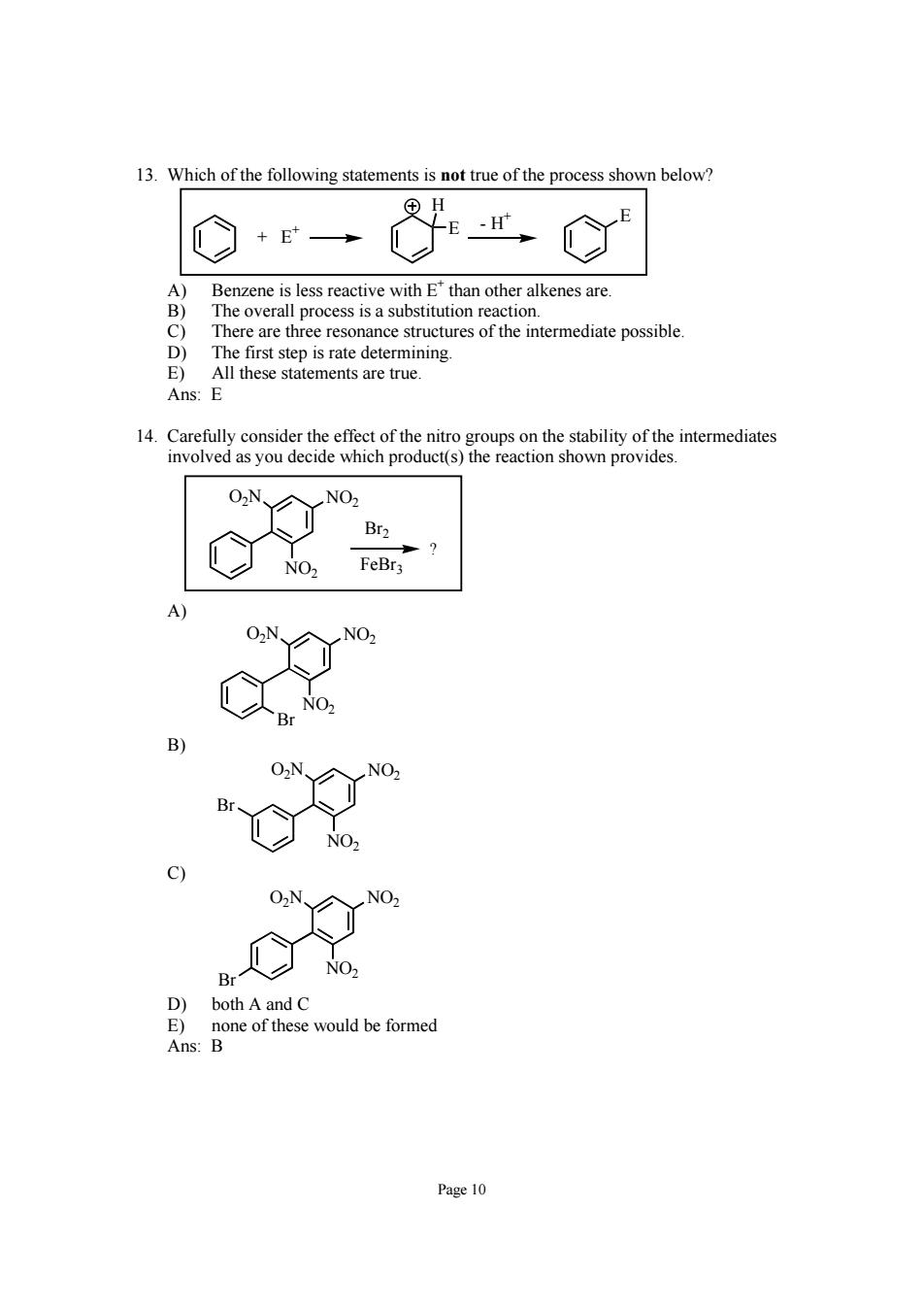
13.Which of the following statements is not true of the process shown below +E→ H Benzene is less reactive with E*than other alkenes are. The overall process is a substitution reaction. There are three resonance structures of the intermediate possible. hese statements are true 0N、 NO, both A and C Ans:Bone o would be formed Page 10
Page 10 13. Which of the following statements is not true of the process shown below? E E H - H+ + E+ A) Benzene is less reactive with E+ than other alkenes are. B) The overall process is a substitution reaction. C) There are three resonance structures of the intermediate possible. D) The first step is rate determining. E) All these statements are true. Ans: E 14. Carefully consider the effect of the nitro groups on the stability of the intermediates involved as you decide which product(s) the reaction shown provides. O2N NO2 NO2 ? Br2 FeBr3 A) O2N NO2 NO2 Br B) O2N NO2 NO2 Br C) O2N NO2 NO2 Br D) both A and C E) none of these would be formed Ans: B