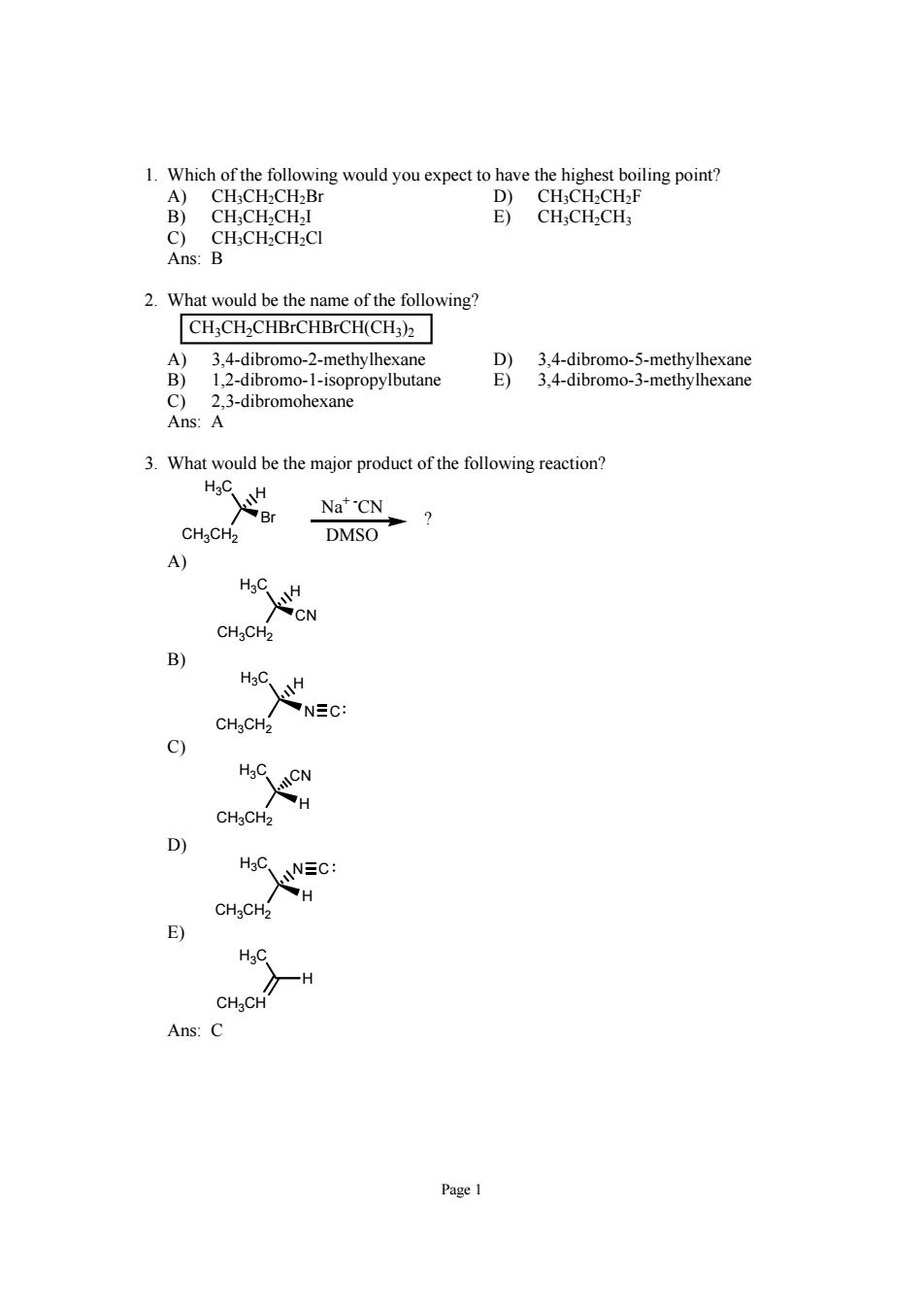
1.Which of the following would you expect to have the highest boiling point? B CH.CH.CH CHCH-CH-C Ans: 2.What would be the name of the following? CH;CH2CHBrCHBrCH(CH3) 3 4-dibromo-2-methylhexane 3 4-dibre 1.2-dibromo-1-isopropylbutane c 2.3-dibromohexane Ans:A 3.What would be the major product of the following reaction? Na"'CN DMSO A) H C. CHgCH2 B) NEC: C) D) CH3CH2 E) H Ans:C
Page 1 1. Which of the following would you expect to have the highest boiling point? A) CH3CH2CH2Br D) CH3CH2CH2F B) CH3CH2CH2I E) CH3CH2CH3 C) CH3CH2CH2Cl Ans: B 2. What would be the name of the following? CH3CH2CHBrCHBrCH(CH3)2 A) 3,4-dibromo-2-methylhexane D) 3,4-dibromo-5-methylhexane B) 1,2-dibromo-1-isopropylbutane E) 3,4-dibromo-3-methylhexane C) 2,3-dibromohexane Ans: A 3. What would be the major product of the following reaction? H3C CH3CH2 Br H Na+ -CN DMSO ? A) H3C CH3CH2 CN H B) H3C CH3CH2 N H C C) H3C CH3CH2 H CN D) H3C CH3CH2 H N C E) H3C CH3CH H Ans: C
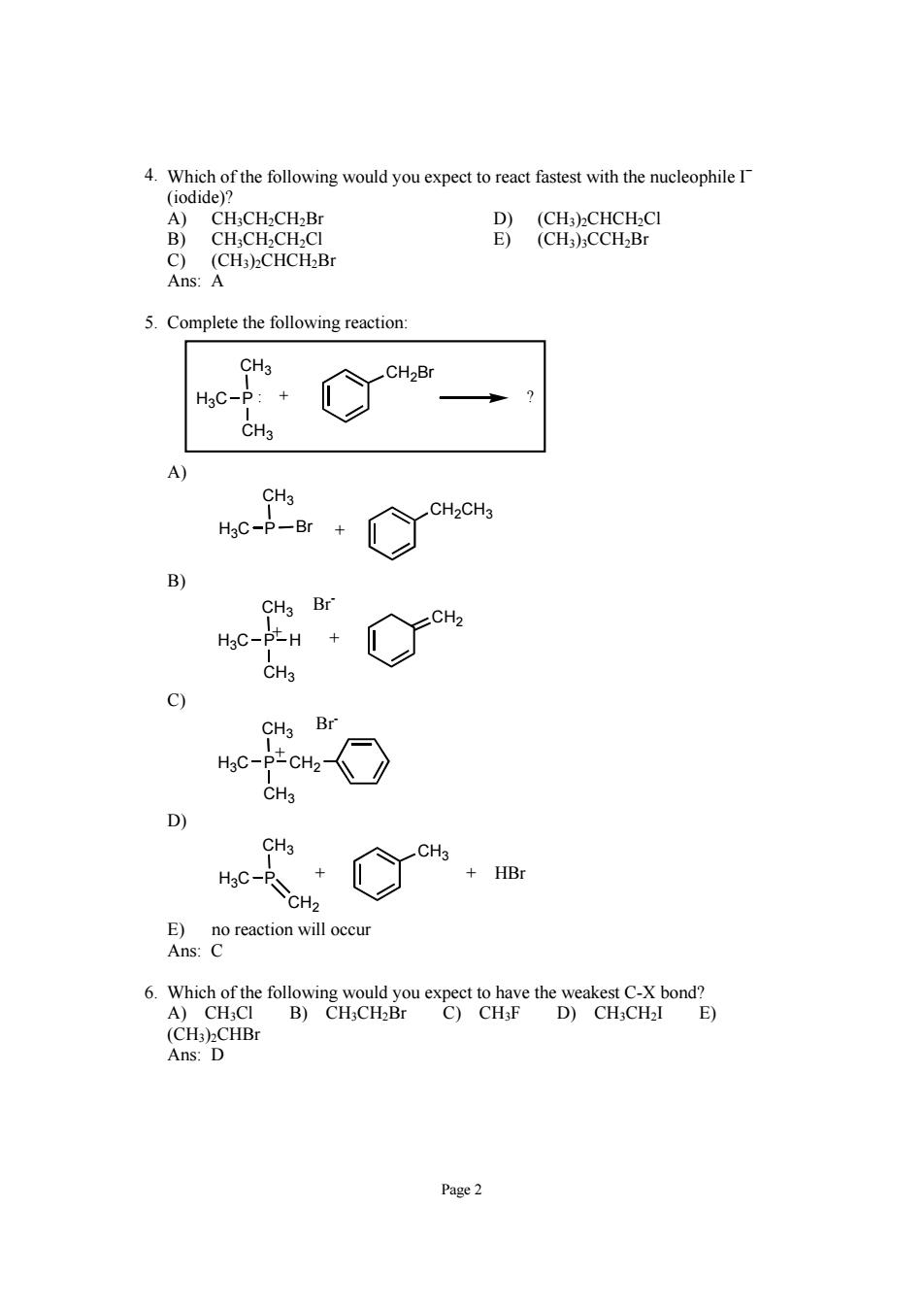
4.Which of the follwing ould youexpet toreact fastest with the nueleophileI (odide H.CH.CH.E CH.CH.CH.CI Ans:A 5.Complete the following reaction: CH3 CH2B H3C-p CH3 CH3 CH2CH3 HgC-P-Br + CH3 Br CH2 HgC-PLH + CH3 9 CH3 Br CH3 CH3 CH3 HC-PSCH2 +HB A)CH-CI D)CHCH2I E) (CH:)2CHBr Ans:D Page2
Page 2 4. Which of the following would you expect to react fastest with the nucleophile I− (iodide)? A) CH3CH2CH2Br D) (CH3)2CHCH2Cl B) CH3CH2CH2Cl E) (CH3)3CCH2Br C) (CH3)2CHCH2Br Ans: A 5. Complete the following reaction: CH3 P CH3 H3C CH2Br : + ? A) CH3 H3C P Br CH2CH3 + B) CH3 P CH3 H3C CH2 H Br- + + C) CH3 P CH3 H3C CH2 Br- + D) CH3 P CH2 H3C CH3 + + HBr E) no reaction will occur Ans: C 6. Which of the following would you expect to have the weakest C-X bond? A) CH3Cl B) CH3CH2Br C) CH3F D) CH3CH2I E) (CH3)2CHBr Ans: D
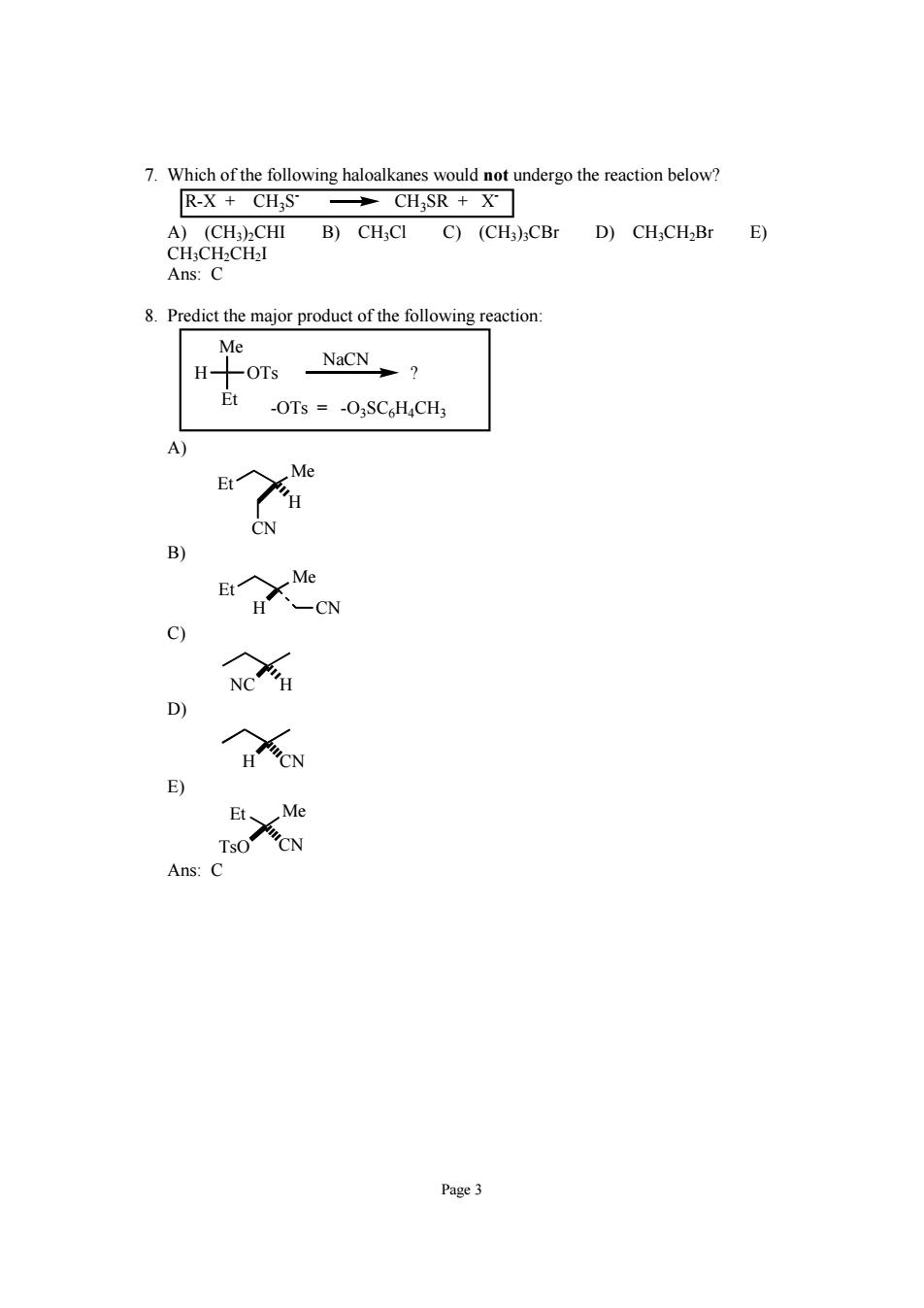
7.Which of the following haloalkanes would not undergo the reaction below? R-X CHS CH SR X A)(CH)2CHⅢI B)CH;CI C)(CH3):CBr D)CH;CH2Br E) CH;CH2CH2I Ans:C 8.Predict the major product of the following reaction Me H -OTs NaCN? Et -OTs =-O3SC H4CH3 H CN C) D) E) Ans:c Page 3
Page 3 7. Which of the following haloalkanes would not undergo the reaction below? R-X + CH3S- CH3SR + X- A) (CH3)2CHI B) CH3Cl C) (CH3)3CBr D) CH3CH2Br E) CH3CH2CH2I Ans: C 8. Predict the major product of the following reaction: H Me OTs Et ? NaCN -OTs = -O3SC6H4CH3 A) Et Me CN H B) Et Me H CN C) NC H D) H CN E) Et Me TsO CN Ans: C
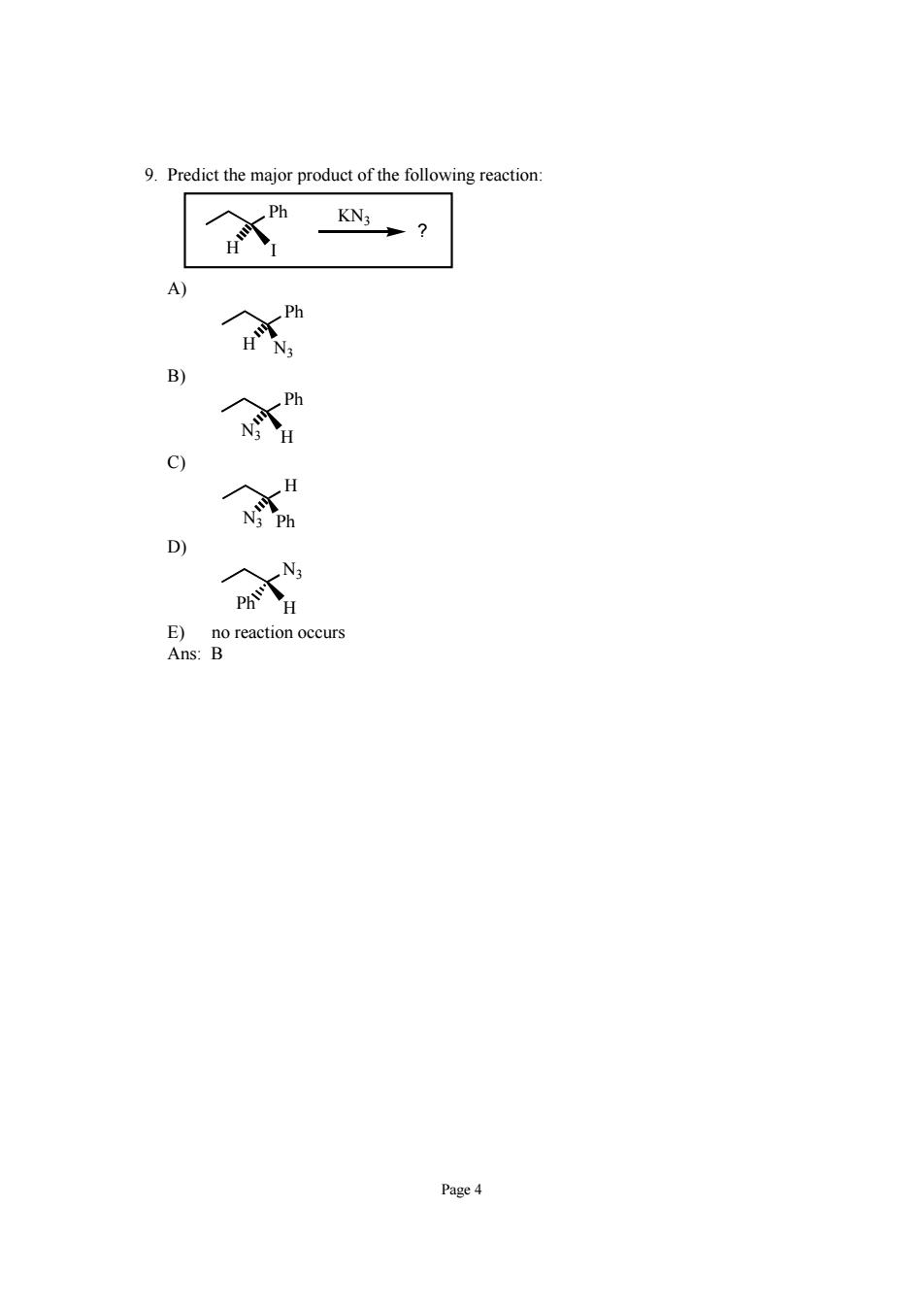
9.Predict the major product of the following reaction 2 c) D) E)no reaction occurs Ans:B Page 4
Page 4 9. Predict the major product of the following reaction: Ph H I KN3 ? A) Ph N3 H B) Ph H N3 C) H N3 Ph D) N3 Ph H E) no reaction occurs Ans: B

10.What is the major product of the following two-step reaction? ○m 1)TsCl,pyridine 2)NaCN C C C) Con ◇o raction cun 11.What is the major product of the following reactior 入入Br Ph,P DMSC0、 A) 入人gm,BP B) c) 人。 D E)no reaction occurs Ans:A Page5
Page 5 10. What is the major product of the following two-step reaction? OH 1) TsCl, pyridine 2) NaCN ? A) O Na B) CN C) OTs D) O E) no reaction occurs Ans: C 11. What is the major product of the following reaction: Br Ph3P / DMSO ? A) PPh3 Br + - B) C) P Br D) E) no reaction occurs Ans: A
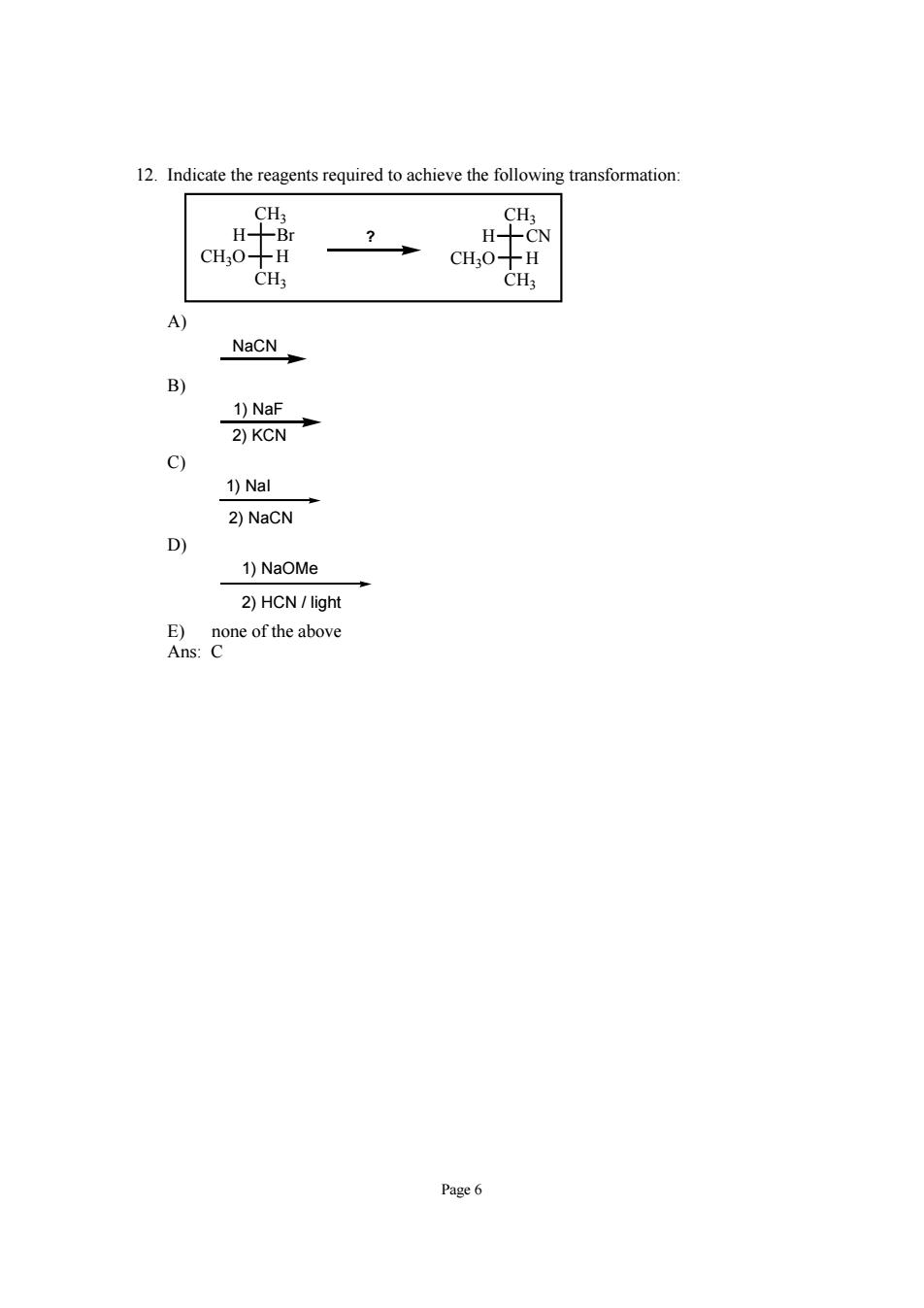
12.Indicate the reagents required to achieve the following transformation -Br HCN CH30-H CH20-H CH3 CH3 A) NaCN B) 1)NaF 2习kcN+ c) 1)Nal 2)NaCN D 1)NaOMe 2)HCN/light coe of the above Page6
Page 6 12. Indicate the reagents required to achieve the following transformation: H CH3 Br CH3 CH3O H H CH3 CN CH3 CH3O H ? A) NaCN B) 1) NaF 2) KCN C) 1) NaI 2) NaCN D) 1) NaOMe 2) HCN / light E) none of the above Ans: C
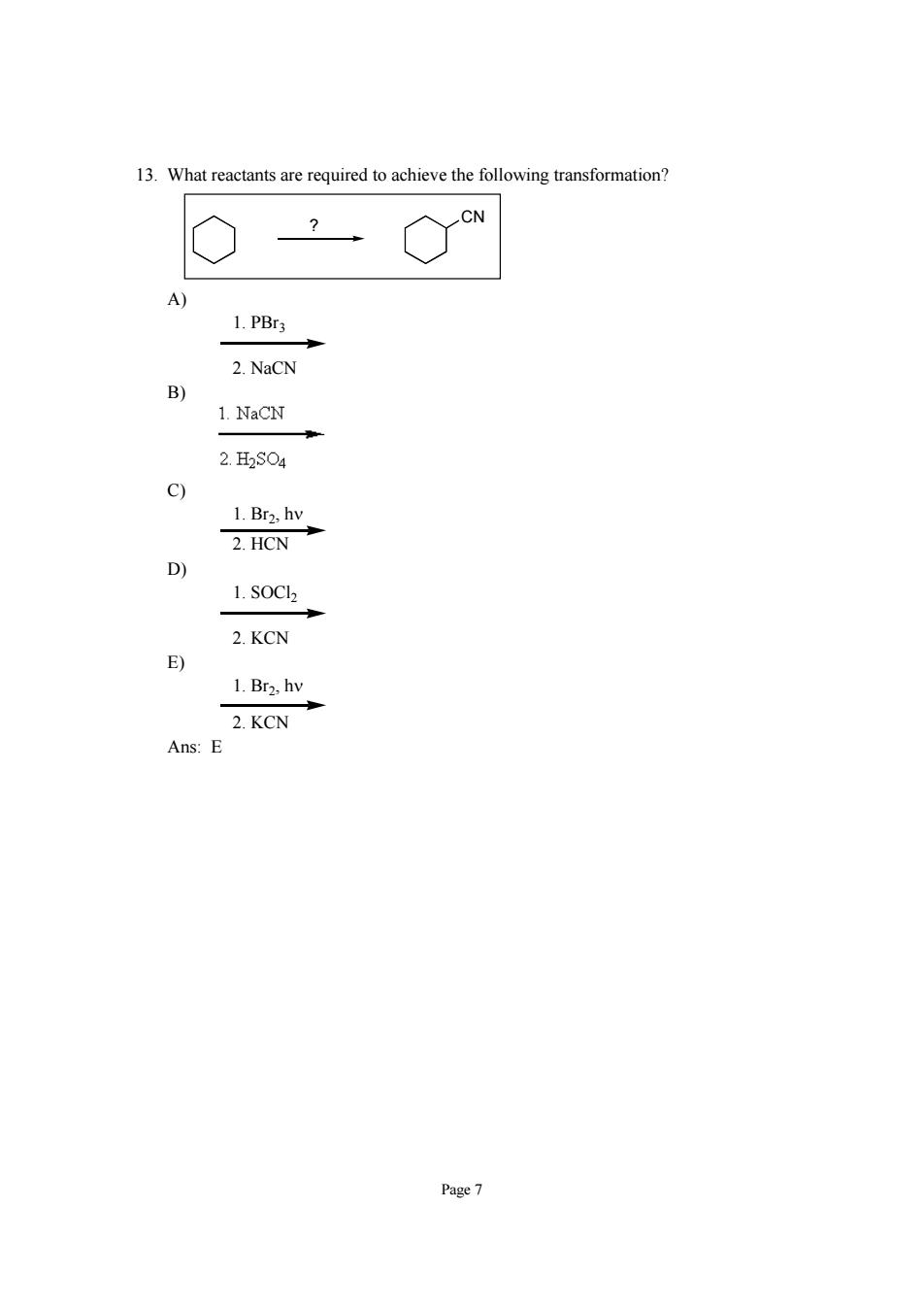
13.What reactants are required to achieve the following transformation? 1.PBrs 2.NaCN 1.NaCN 2.H2S04 2.HCN 1.s0C2 2.KCN 1.Br2,hv 2.KCN Ans:E Page7
Page 7 13. What reactants are required to achieve the following transformation? CN ? A) 1. PBr3 2. NaCN B) C) 1. Br2, hν 2. HCN D) 1. SOCl2 2. KCN E) 1. Br2, hν 2. KCN Ans: E

14.What reactants are required to achieve the following transformation? ? o A) NaCN 1)NaOH 2)KCN S-CH3 c-一pnoine 2.KCN 1)Br2/light 2)NaCN E)none of the above Ans:C 15.To which side (if any)would the following equilibrium lie? CH;CH2SK*+HOH CHCH2SH KOH to the right t and lef E)only SN2.SNI and E2 reactions are possible Ans:A 16.Several alkyl halides,including iodomethane,are known carcinogens or cancer-suspect materials To destroy these materials by conversion to non-ele can B)NHs D)Nal E)CH3CO2H Ans:B 17.Which of the following is not normally a good leaving group on carbon? A)Br B)OCH3 C)CI D)OSO2R E)I Ans:B Page 8
Page 8 14. What reactants are required to achieve the following transformation? OH CN ? A) NaCN B) 1) NaOH 2) KCN C) 1. 2. KCN Cl S O O CH3 , pyridine D) 1) Br2 / light 2) NaCN E) none of the above Ans: C 15. To which side (if any) would the following equilibrium lie? CH3CH2S- K+ + HOH CH3CH2SH + KOH A) to the left B) to the right C) equally to the right and left D) there is no way to tell E) only SN2, SN1 and E2 reactions are possible Ans: A 16. Several alkyl halides, including iodomethane, are known carcinogens or cancer-suspect materials. To destroy these materials by conversion to non-electrophilic species, you can react them with nucleophiles. Which of the following would be the best for rapidly destroying methyl iodide (iodomethane)? A) CH3OH B) NH3 C) H2O D) NaI E) CH3CO2H Ans: B 17. Which of the following is not normally a good leaving group on carbon? A) Br B) OCH3 C) Cl D) OSO2R E) I Ans: B

18.Predict the major product of the following reaction: Br NaN3? B) 心 c) b) C 19.Which oft A Ans:D Page9
Page 9 18. Predict the major product of the following reaction: Br NaN3 ? A) N3 B) C) D) E) no reaction occurs Ans: E 19. Which of the following reagents would best accomplish a typical SN2 reaction? A) CH3OH B) H2O C) HCN D) KCN E) KOt Bu Ans: D
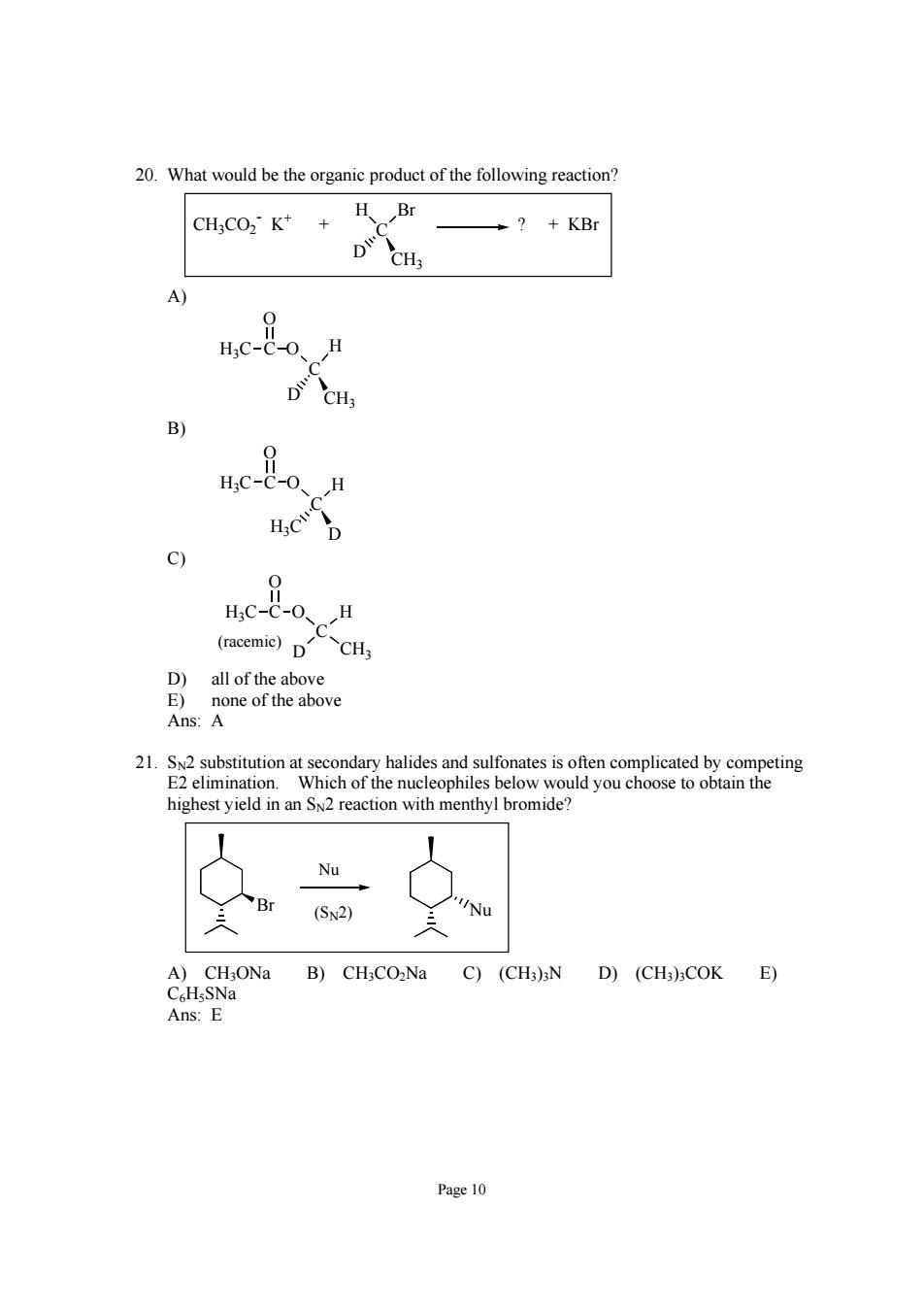
20.What would be the organic product of the following reaction? CH:CO2 K++ H Br ?KBr DCHs B) HCD 9 HC-C-0、H (racemie)DCH D)all of the above E)none of the above Ans:A 21.2 substitution at seco condary halidesandsulfismlice by competing n an tion wi menthyl mide? (SN2) 'Nu )CH;ONa B)CH;CO2Na C)(CH3)N D)(CH3)COK E) Ans: Page 10
Page 10 20. What would be the organic product of the following reaction? C CH3 D H Br CH3CO2 - K+ + ? + KBr A) C CH3 D C O H O H3C B) C D H3C C O H O H3C C) C D CH3 C O H O H3C (racemic) D) all of the above E) none of the above Ans: A 21. SN2 substitution at secondary halides and sulfonates is often complicated by competing E2 elimination. Which of the nucleophiles below would you choose to obtain the highest yield in an SN2 reaction with menthyl bromide? Br Nu Nu (SN2) A) CH3ONa B) CH3CO2Na C) (CH3)3N D) (CH3)3COK E) C6H5SNa Ans: E