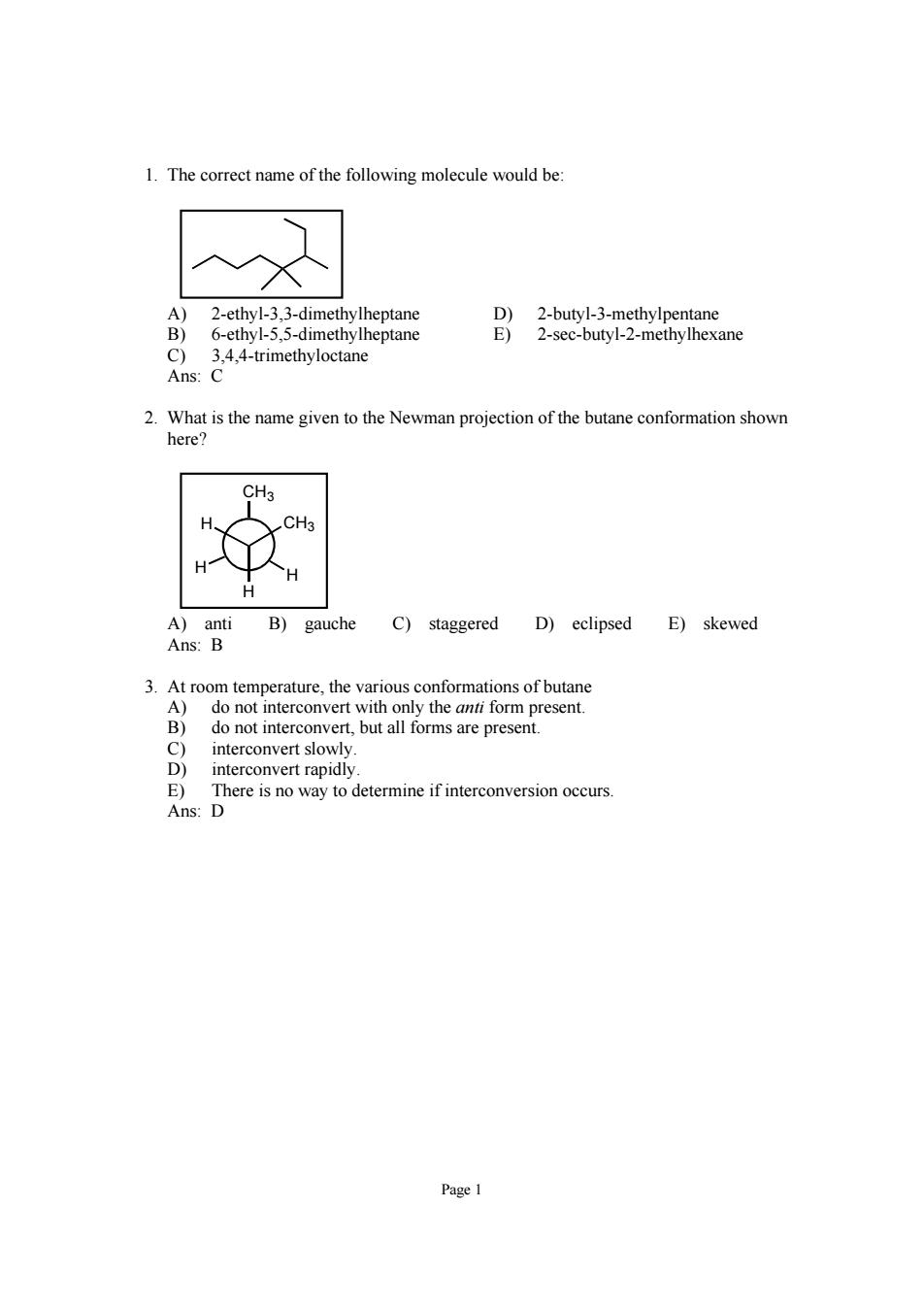
1.The correct name of the following molecule would be A)2-ethyl-3.3-dimethylheptane B)6-ethyl-5,5-dimethylheptane cane C) 3,4,4-trimethyloctane Ans:C 2.What is the name given to the Newman p butane conformation show CH A)anti B)gauche C)staggered D)eclipsed E)skewed Ans:B 3.At room temperature,the various conformations of butane B not inte E)There is no way to determine if interconversion occurs Ans:D Page
Page 1 1. The correct name of the following molecule would be: A) 2-ethyl-3,3-dimethylheptane D) 2-butyl-3-methylpentane B) 6-ethyl-5,5-dimethylheptane E) 2-sec-butyl-2-methylhexane C) 3,4,4-trimethyloctane Ans: C 2. What is the name given to the Newman projection of the butane conformation shown here? H H CH3 CH3 H H A) anti B) gauche C) staggered D) eclipsed E) skewed Ans: B 3. At room temperature, the various conformations of butane A) do not interconvert with only the anti form present. B) do not interconvert, but all forms are present. C) interconvert slowly. D) interconvert rapidly. E) There is no way to determine if interconversion occurs. Ans: D
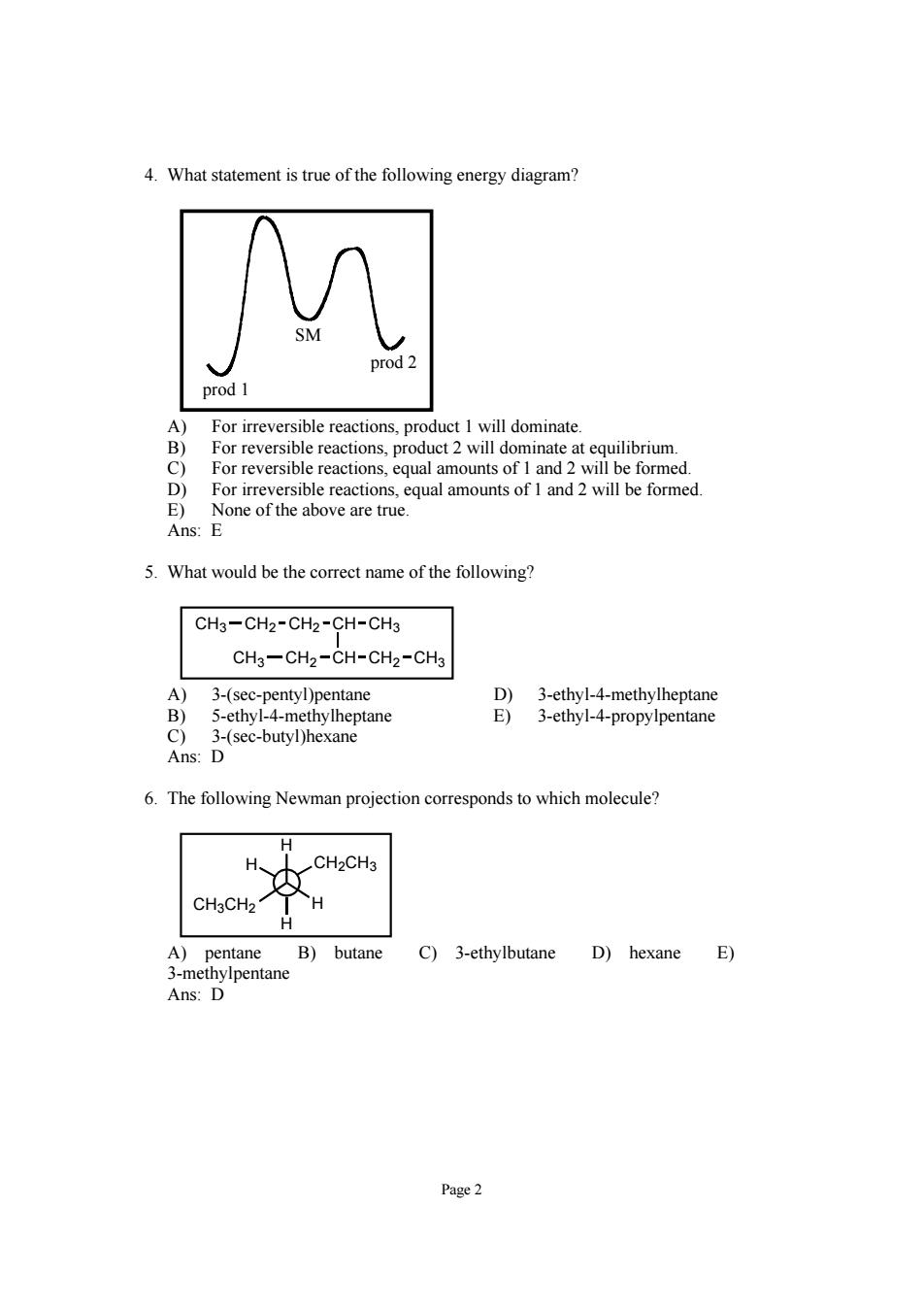
4.What statement is true of the following energy diagram? SM prod 1 For irreversible reaction dominate at for re eversible reactions.equal amounts of l and 2 will be formed D) For irreversible reactions,equal amounts of 1 and 2 will be formed. E) None of the above are true. Ans:E 5.What would be the correct name of the following CH3-CH2-CH2-CH-CH3 CH3-CH2-CH-CH2-CH 3-(se pentane 6.The following Newman projection corresponds to which molecule? H CH2CH3 CH3CH2 A)pentane B)butane C)3-ethylbutane D)hexane E) Page2
Page 2 4. What statement is true of the following energy diagram? prod 1 prod 2 SM A) For irreversible reactions, product 1 will dominate. B) For reversible reactions, product 2 will dominate at equilibrium. C) For reversible reactions, equal amounts of 1 and 2 will be formed. D) For irreversible reactions, equal amounts of 1 and 2 will be formed. E) None of the above are true. Ans: E 5. What would be the correct name of the following? CH2 CH2 CH CH3 CH3 CH2 CH CH2 CH3 CH3 A) 3-(sec-pentyl)pentane D) 3-ethyl-4-methylheptane B) 5-ethyl-4-methylheptane E) 3-ethyl-4-propylpentane C) 3-(sec-butyl)hexane Ans: D 6. The following Newman projection corresponds to which molecule? H CH3CH2 H H CH2CH3 H A) pentane B) butane C) 3-ethylbutane D) hexane E) 3-methylpentane Ans: D
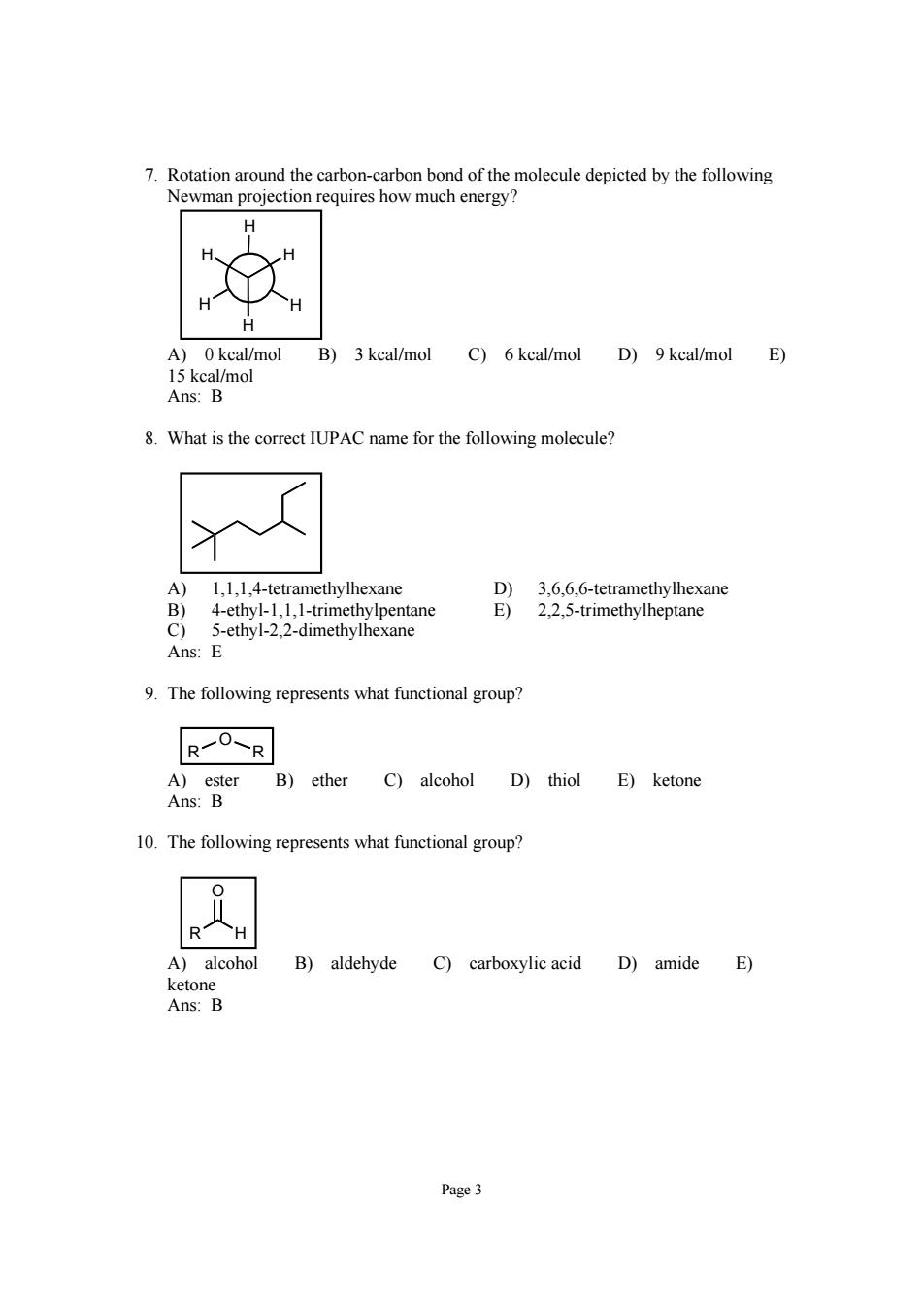
7.Rotation around the carbon- arbon bond of the molecule depicted by the following Newman projection requires how much energy A)0kcal/mol B)3 kcal/mol C)6 keal/mol D)9 kcal/mol E) 8.What is the correct IUPAC name for the following molecule? A) 1,1,1,4-tetramethylhexane D)3.6.6.6-tetramethylhexane 4-ethyl-1,1,1-trimethylpentane E)2,2,5-trimethylheptane -ethyl-2,2-dimethylhexane Ans: 9.The following represents what functional group? R7O-R A)ester B)ether C)alcohol D)thiol E)ketone Ans:B 10.The following represents what functional group? A)alcohol B)aldehyde C)carboxylic acid D)amide E) Page3
Page 3 7. Rotation around the carbon-carbon bond of the molecule depicted by the following Newman projection requires how much energy? H H H H H H A) 0 kcal/mol B) 3 kcal/mol C) 6 kcal/mol D) 9 kcal/mol E) 15 kcal/mol Ans: B 8. What is the correct IUPAC name for the following molecule? A) 1,1,1,4-tetramethylhexane D) 3,6,6,6-tetramethylhexane B) 4-ethyl-1,1,1-trimethylpentane E) 2,2,5-trimethylheptane C) 5-ethyl-2,2-dimethylhexane Ans: E 9. The following represents what functional group? R R O A) ester B) ether C) alcohol D) thiol E) ketone Ans: B 10. The following represents what functional group? R H O A) alcohol B) aldehyde C) carboxylic acid D) amide E) ketone Ans: B

11.The following represents what functional group? R-CEN B)thiol C)ketone D)nitrile E)amine 12.What is the correct structure for tertbutyl bromide? A) B) 丫e 人 Page4
Page 4 11. The following represents what functional group? R C N A) amide B) thiol C) ketone D) nitrile E) amine Ans: D 12. What is the correct structure for tertbutyl bromide? A) Br B) Br C) Br D) Br E) Br Ans: B

13.The following Newman projection represents which molecule? CH3 CH2CH3 CH2CH2CH3 Page5
Page 5 13. The following Newman projection represents which molecule? H H3CH2CH2C CH3 CH2CH3 CH3 CH2CH2CH3 A) B) CH3 CH3 C) D) E) Ans: A
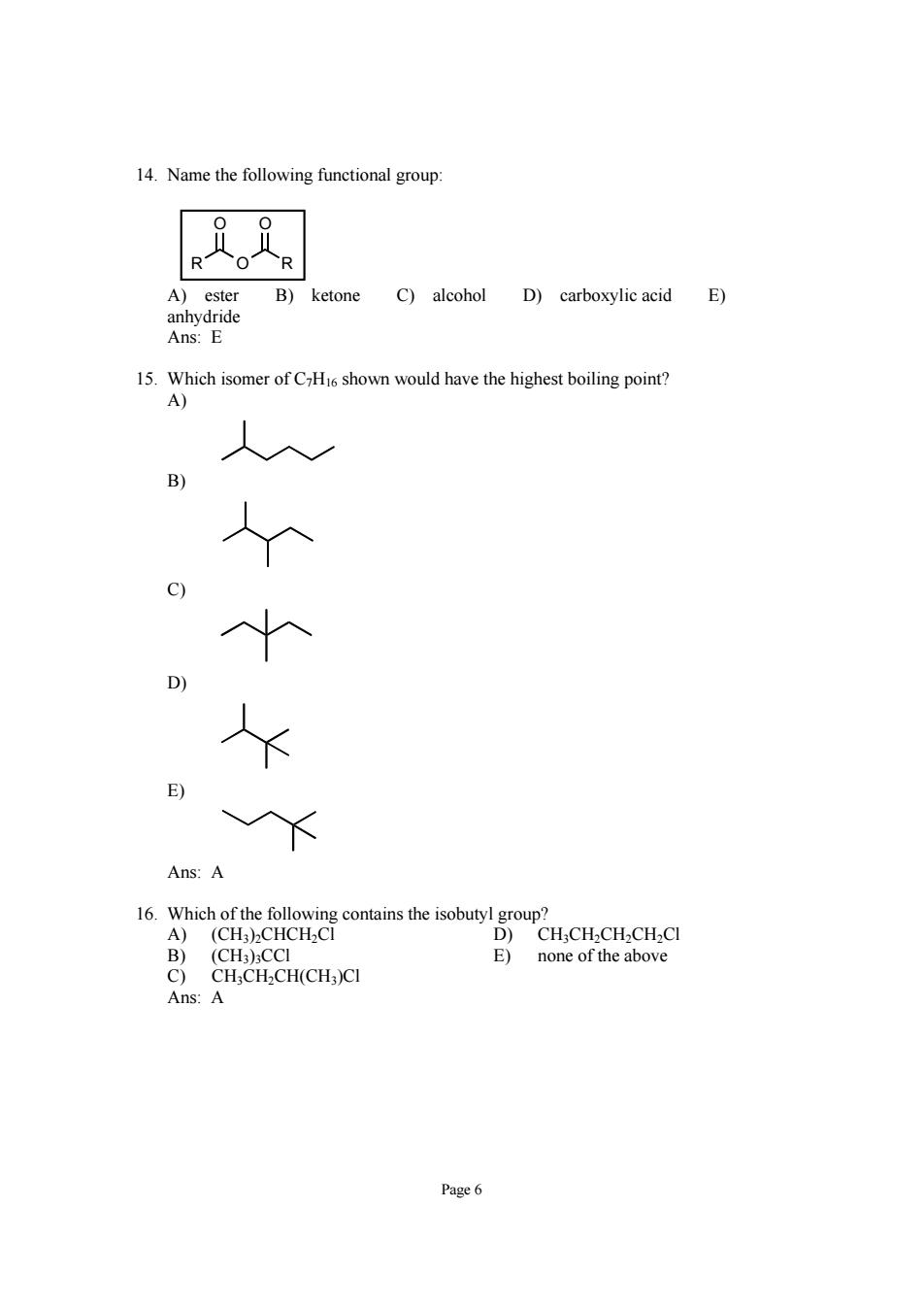
14.Name the following functional group: R R ketone C)alcohol D)carboxylic acid E) 15.Which isomer of CHis shown would have the highest boiling point? A) Ans:A D)CH.CH.CH.CH.CI (CH3)CCI E)none of the above C)CH;CH2CH(CH)CI Ans:A Page6
Page 6 14. Name the following functional group: ROR O O A) ester B) ketone C) alcohol D) carboxylic acid E) anhydride Ans: E 15. Which isomer of C7H16 shown would have the highest boiling point? A) B) C) D) E) Ans: A 16. Which of the following contains the isobutyl group? A) (CH3)2CHCH2Cl D) CH3CH2CH2CH2Cl B) (CH3)3CCl E) none of the above C) CH3CH2CH(CH3)Cl Ans: A

17.Which of the follo H.CH.CH.CH.CI CHCH-CH(CH3)CI AnsC 18.How would the following molecule be properly named? CH3 CH3-CH CH3CH2-CH HC-CH2CH2CH3 CH2 CHa 3-ethyl-2-methyl-4- D)3-isopropyl-4-propylhexane B) opyiepne 3.4-diethyl-2-methylheptane E)none of the above 19. p) 24.4-trimethylheptane 3-tert-butyl-2.2-dimethylbutane none of the above C) 2.2,3,4,4-pentamethylpentane Ans:C 20.What is the smalles C)pentane E)all are liquids 21.All steroids are derivatives of the ring system shown.How many tertiary hydrogens are in this ring system? 〔 EeB剧24D)5目6 Page7
Page 7 17. Which of the following contains the sec-butyl group? A) (CH3)2CHCH2Cl D) CH3CH2CH2CH2Cl B) (CH3)3CCl E) None of the above C) CH3CH2CH(CH3)Cl Ans: C 18. How would the following molecule be properly named? CH3 CH CH CH3 HC CH2 CH3 CH3CH2 CH2CH2CH3 A) 3-ethyl-2-methyl-4-propylhexane D) 3-isopropyl-4-propylhexane B) 3,4-diethyl-2-methylheptane E) none of the above C) 4-ethyl-3-isopropylheptane Ans: B 19. The IUPAC name for [(CH3)3C]2CHCH3 is: A) 1,1-di-tert-butylethane D) 2,4,4-trimethylheptane B) 3-tert-butyl-2,2-dimethylbutane E) none of the above C) 2,2,3,4,4-pentamethylpentane Ans: C 20. What is the smallest alkane that is liquid at room temperature? A) propane B) butane C) pentane D) hexane E) all are liquids Ans: C 21. All steroids are derivatives of the ring system shown. How many tertiary hydrogens are in this ring system? A) none B) 2 C) 4 D) 5 E) 6 Ans: E

22.What is the correct IUPAC name for the following molecule: )2-ethyl-2.6-dimethylheptane D)2.6,6-trimethyloctane B) 1,1,5,5-tetramethylhexane E)4,4-dimethyl-1-isopropylhexane Ans: -etramethytheptane 23.What is the name given to the Newman projection of the butane conformation shown here? CHa A)eclipsed B) gauche C)staggered D)anti E)skewed Ans:D 24.Calculate AG°for the following reaction at25℃and△S-0 CH4 Br2 CHBr HBr Bond Ave Strengt地 C-C 83 (kcal/mol 99 (kcal/mol Br-Br 46(keal/mol) -34 kcal/r none of the above Ans:A 25.Which of the following has the highest pKa? A)HI B)NH3 C)HNO3 D)CHsCOOH E)H2SO4 Ans:B Page 8
Page 8 22. What is the correct IUPAC name for the following molecule: A) 2-ethyl-2,6-dimethylheptane D) 2,6,6-trimethyloctane B) 1,1,5,5-tetramethylhexane E) 4,4-dimethyl-1-isopropylhexane C) 1,1,5,5-tetramethylheptane Ans: D 23. What is the name given to the Newman projection of the butane conformation shown here? CH3 H H CH3 H H A) eclipsed B) gauche C) staggered D) anti E) skewed Ans: D 24. Calculate ΔGo for the following reaction at 25 o C and ΔS=0 A) –10 kcal/mol D) –34 kcal/mol B) 10 kcal/mol E) none of the above C) 34 kcal/mol Ans: A 25. Which of the following has the highest pKa? A) HI B) NH3 C) HNO3 D) CH3COOH E) H2SO4 Ans: B
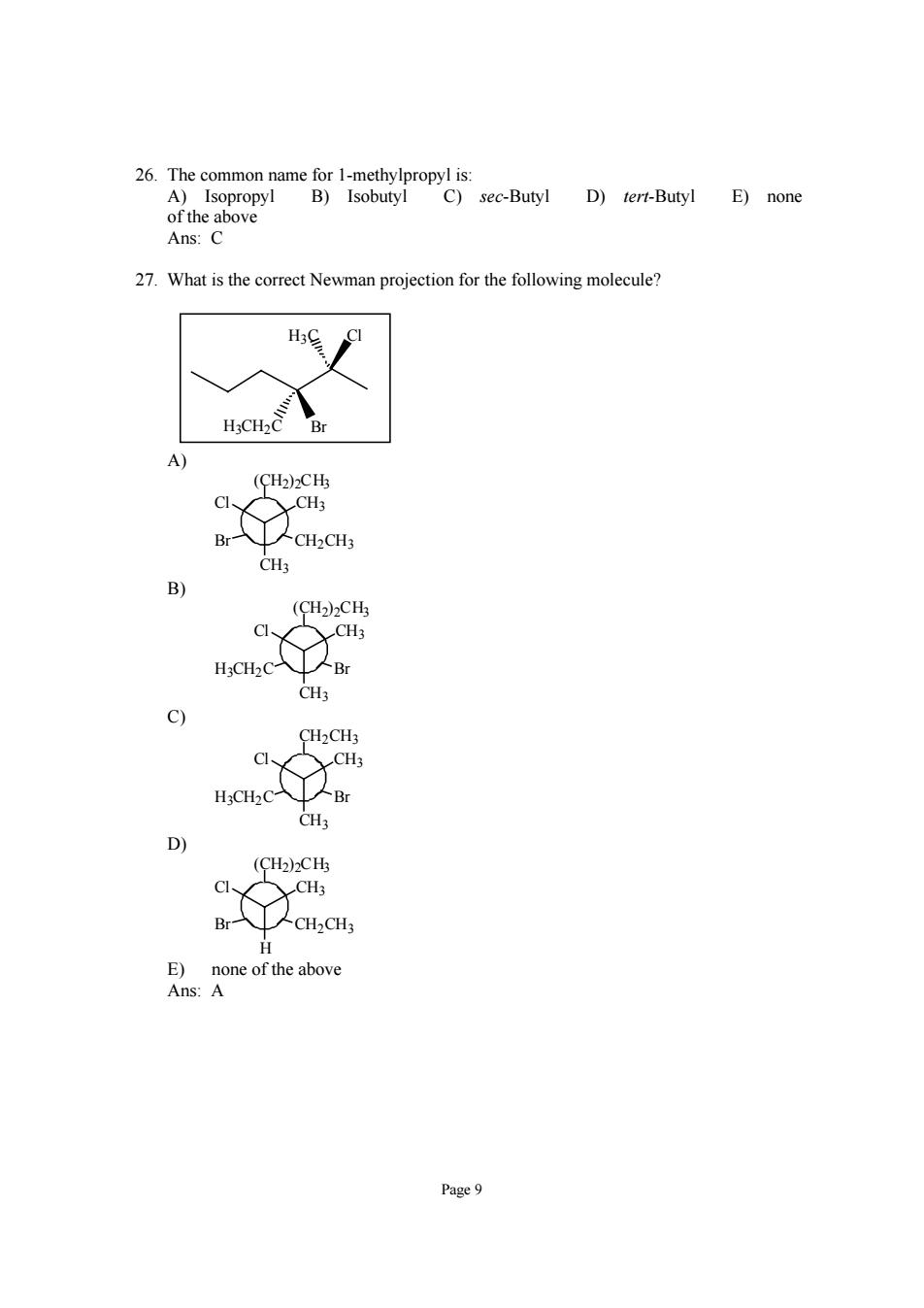
Ans:C 27.What is the correct Newman projection for the following molecule? H3CH2C (CH2)2CHz C、 CH3 (CH2)2CH H:CH2C- Br CH3 c) CH2CH3 CH3 H;CH2C CH b) (CH2)2CHs C、 CH3 -CH2CH E)none of the above Ans:A Page9
Page 9 26. The common name for 1-methylpropyl is: A) Isopropyl B) Isobutyl C) sec-Butyl D) tert-Butyl E) none of the above Ans: C 27. What is the correct Newman projection for the following molecule? H3C Cl H3CH2C Br A) CH3 Cl CH3 (CH2)2CH3 Br CH2CH3 B) CH3 Cl CH3 (CH2)2CH3 H3CH2C Br C) CH3 Cl CH3 CH2CH3 H3CH2C Br D) H Cl CH3 (CH2)2CH3 Br CH2CH3 E) none of the above Ans: A
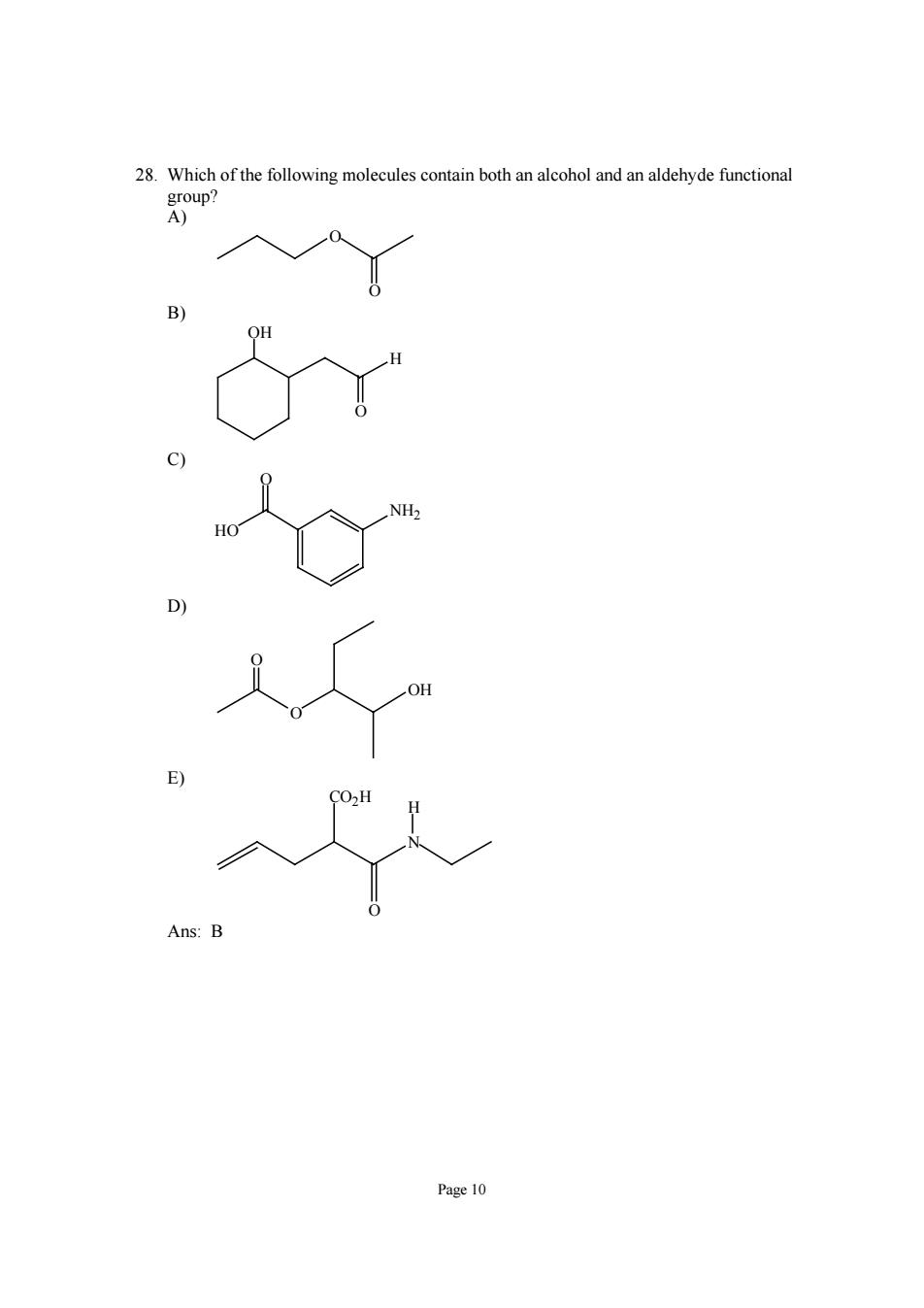
28.Which of the following molecules contain both an alcohol and an aldehyde functional oup? Page 10
Page 10 28. Which of the following molecules contain both an alcohol and an aldehyde functional group? A) O O B) H O OH C) HO NH2 O D) O OH O E) N CO2H O H Ans: B