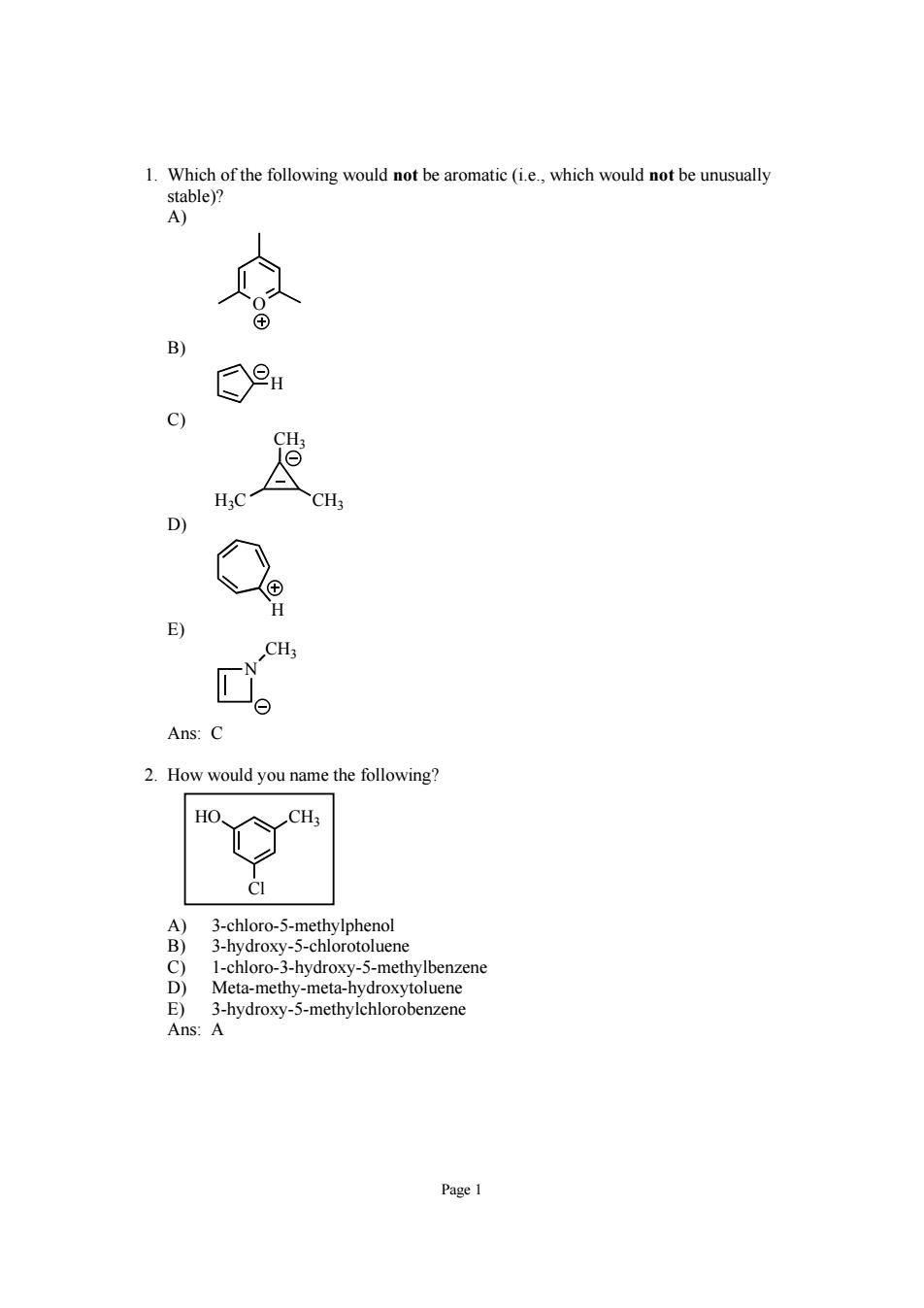
1.Which of the following would not be aromatic (i.e..which would not be unusually 0 CH Ans:C 2.How would you name the following? HO CH; 8 1-chloro-3-hydroxy-5-methylbenzene Meta-methy-meta-hydroxytoluene 3-hydroxy-5-methylchlorobenzene Ans:A Page 1
Page 1 1. Which of the following would not be aromatic (i.e., which would not be unusually stable)? A) O B) H C) H3C CH3 CH3 D) H E) N CH3 Ans: C 2. How would you name the following? HO CH3 Cl A) 3-chloro-5-methylphenol B) 3-hydroxy-5-chlorotoluene C) 1-chloro-3-hydroxy-5-methylbenzene D) Meta-methy-meta-hydroxytoluene E) 3-hydroxy-5-methylchlorobenzene Ans: A
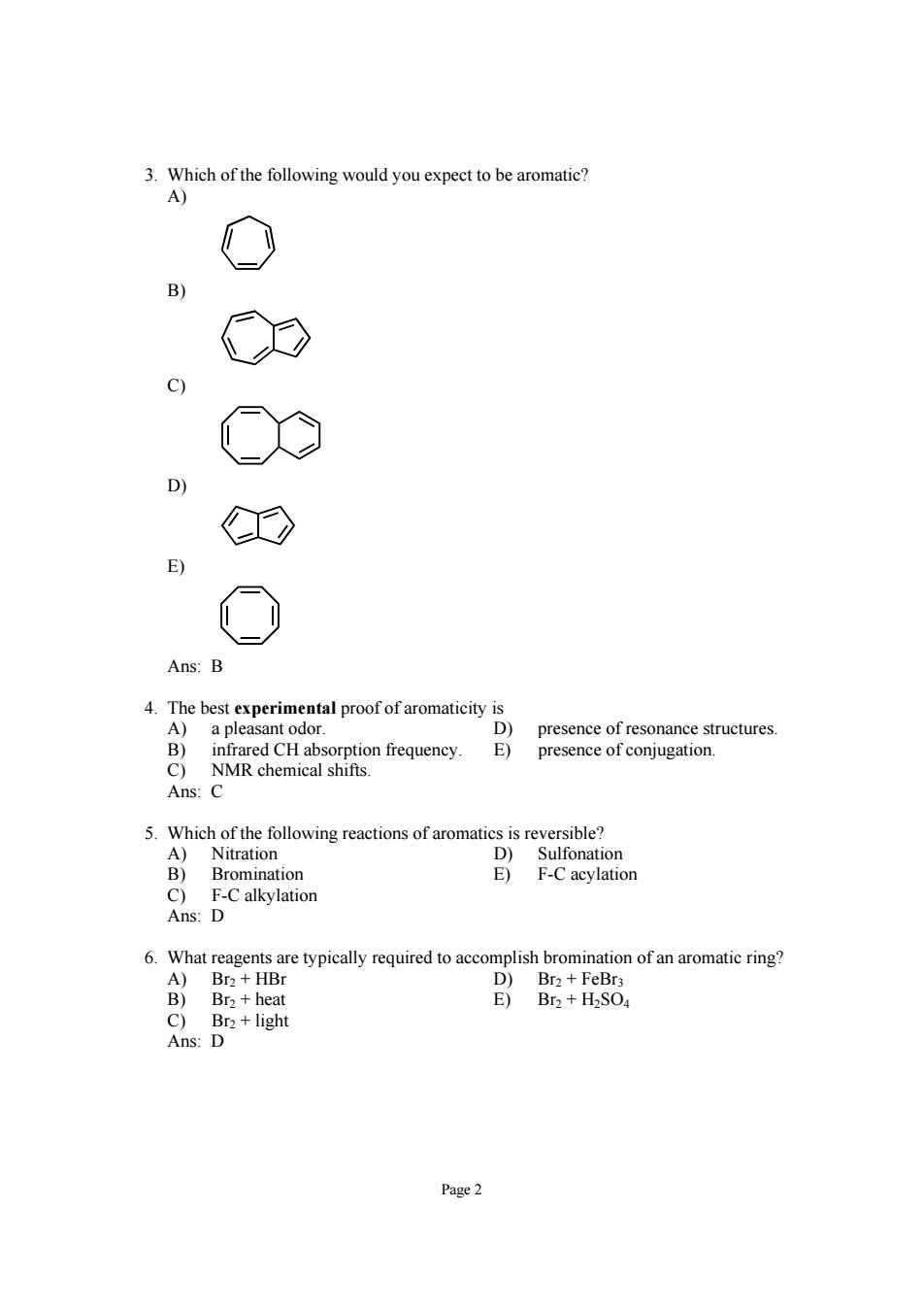
3.Which of the following would you expect to be aromatic? A) C E Ans:B 兰fof红mi presence of resonance structures presence of conjugation Ans: 5.Which of the following reactions of aromatics is reversible? A)Nitration D)Sulfonation B)Bromination F-C acylation 6.What reagents are typically required to accomplish bromination of an aromatic ring? A)Br2+HBr D)Br2+FeBrs B)Br2+heat E)Br2+H2SOa C)Br2+light Ans:D Page2
Page 2 3. Which of the following would you expect to be aromatic? A) B) C) D) E) Ans: B 4. The best experimental proof of aromaticity is A) a pleasant odor. D) presence of resonance structures. B) infrared CH absorption frequency. E) presence of conjugation. C) NMR chemical shifts. Ans: C 5. Which of the following reactions of aromatics is reversible? A) Nitration D) Sulfonation B) Bromination E) F-C acylation C) F-C alkylation Ans: D 6. What reagents are typically required to accomplish bromination of an aromatic ring? A) Br2 + HBr D) Br2 + FeBr3 B) Br2 + heat E) Br2 + H2SO4 C) Br2 + light Ans: D
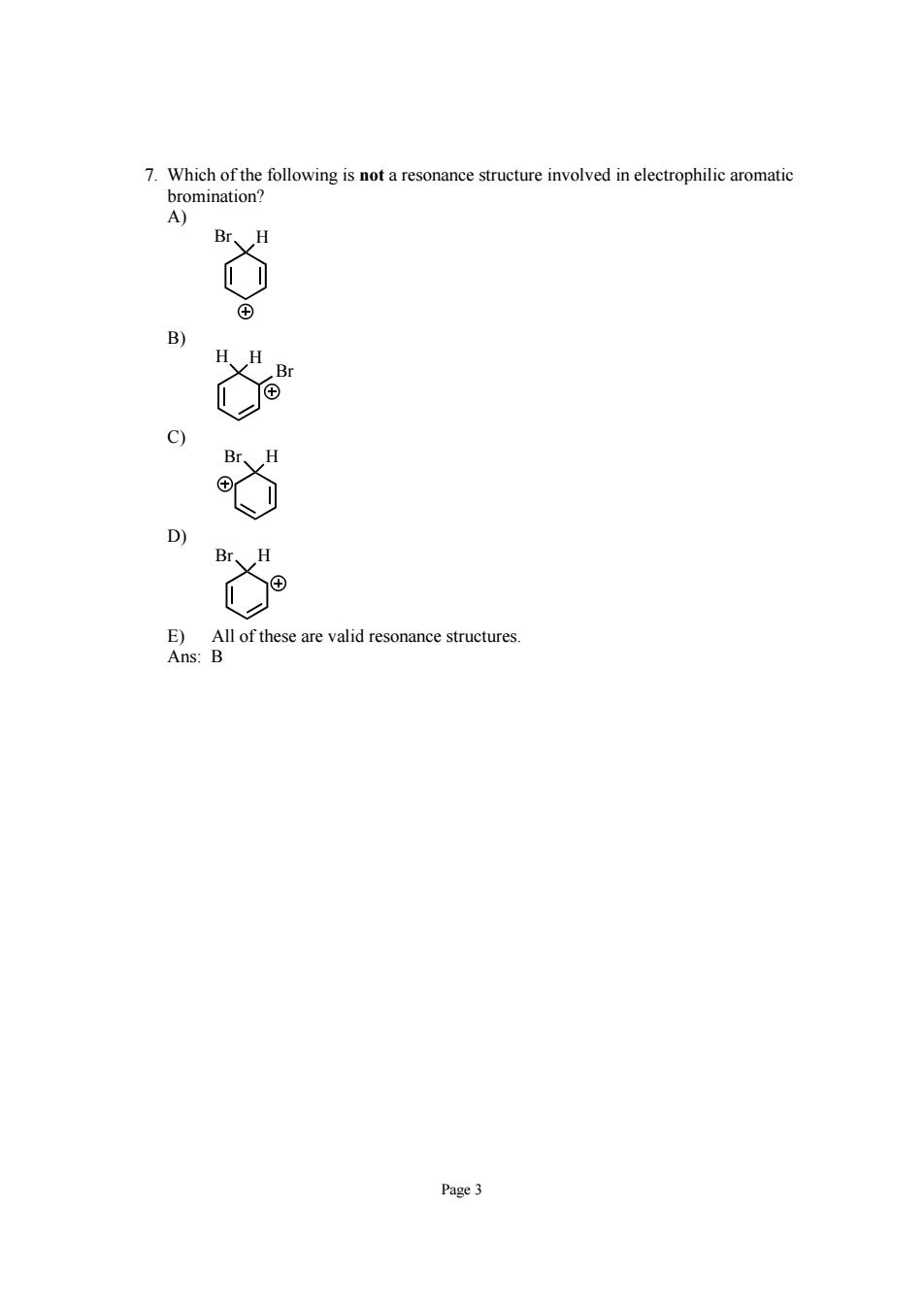
7.Which of the following is not a resonance structure involved in electrophilic aromatic B) c) ll of these are valid resonance Page3
Page 3 7. Which of the following is not a resonance structure involved in electrophilic aromatic bromination? A) Br H B) Br H H C) Br H D) Br H E) All of these are valid resonance structures. Ans: B

8.What would be the product of the following reaction? 1.BH吟HF CH3 2.H2O2,NaOH OH ne)-cn s◇a HO E)No reaction occurs Ans:E Page4
Page 4 8. What would be the product of the following reaction? CH3 H3C ? 1. BH3昑HF 2. H2O2, NaOH A) CH3 H3C OH B) CH3 H3C OH C) OH CH3 H3C D) CH3 H3C HO E) No reaction occurs Ans: E
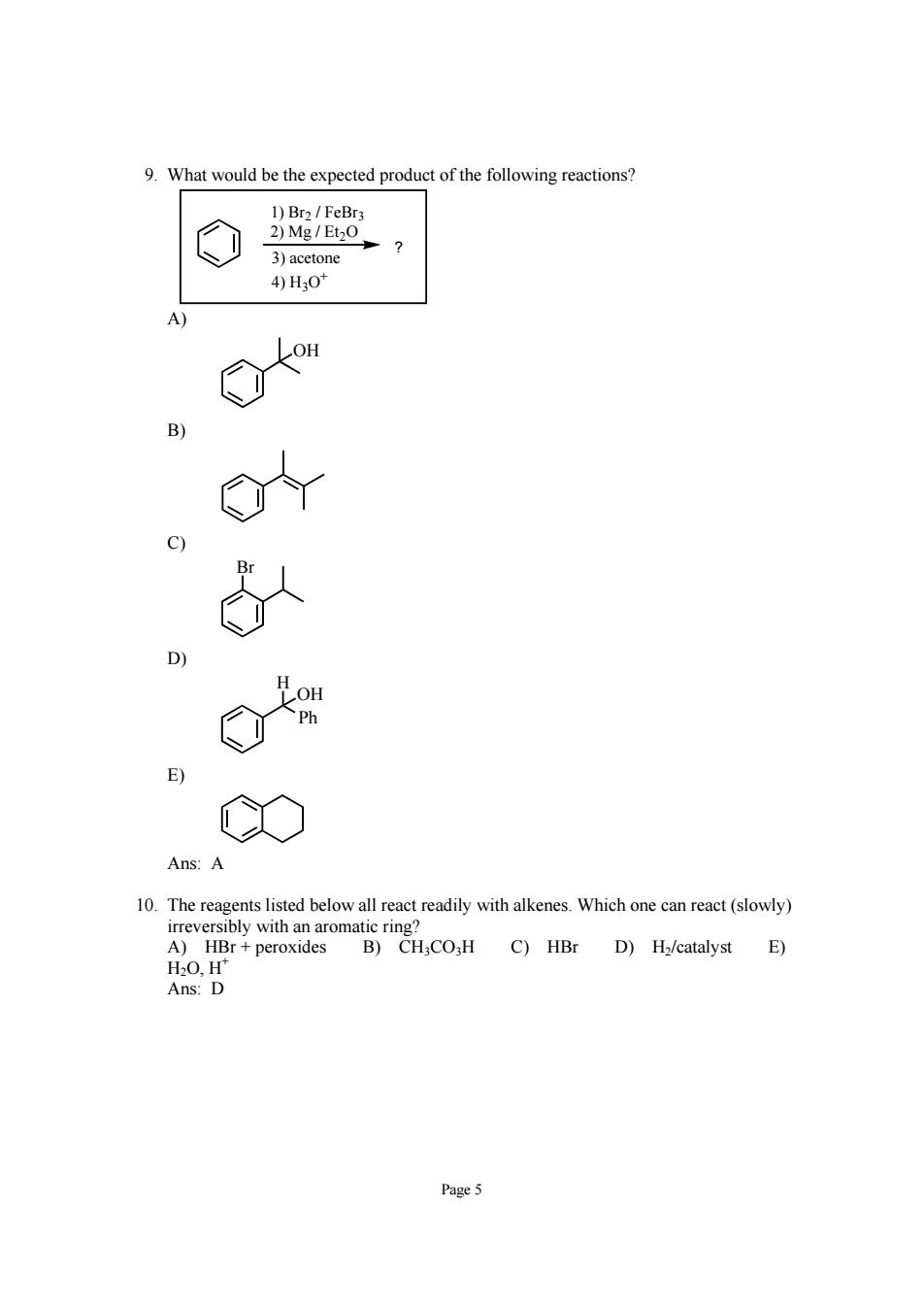
9.What would be the expected product of the following reactions? 1)Br2/FeBrs 2)Mg/Et2O 3)acetone 4)H30* 3 Ans:A A)Hrperoxides B)CH,CO,H C)HBr D)H/catalyst E) H20,H Ans:D Page5
Page 5 9. What would be the expected product of the following reactions? ? 3) acetone 4) H3O+ 2) Mg / Et2O 1) Br2 / FeBr3 A) OH B) C) Br D) OH Ph H E) Ans: A 10. The reagents listed below all react readily with alkenes. Which one can react (slowly) irreversibly with an aromatic ring? A) HBr + peroxides B) CH3CO3H C) HBr D) H2/catalyst E) H2O, H+ Ans: D

11.How many mononitrated (on the aromatic ring)derivatives of meta-xylene(= le?(Don't worry about how they might be made or 12.Which of the reagents given below would accomplish the reaction shown? ON、 CH ON、 CO2H 含c&Oa 日nce C)CICO2H,AICI; Ans:B 13.Which of the following would you expect to react fastest in an S reaction(consider B) C c) Br Page6
Page 6 11. How many mononitrated (on the aromatic ring) derivatives of meta-xylene (= 1,3-dimethylbenzene) are possible? (Don't worry about how they might be made or which would be the major product.) A) 1 B) 2 C) 3 D) 4 E) 5 Ans: C 12. Which of the reagents given below would accomplish the reaction shown? CH3 CH3 O2N CO2H CO2H O2N ? A) H2O2, KOH, heat D) O3, then H2O B) Na2Cr2O7, H+ , heat E) none of these C) ClCO2H, AlCl3 Ans: B 13. Which of the following would you expect to react fastest in an SN1 reaction (consider the mechanism)? A) Br B) Br C) Br D) all would react rapidly E) none would react Ans: A
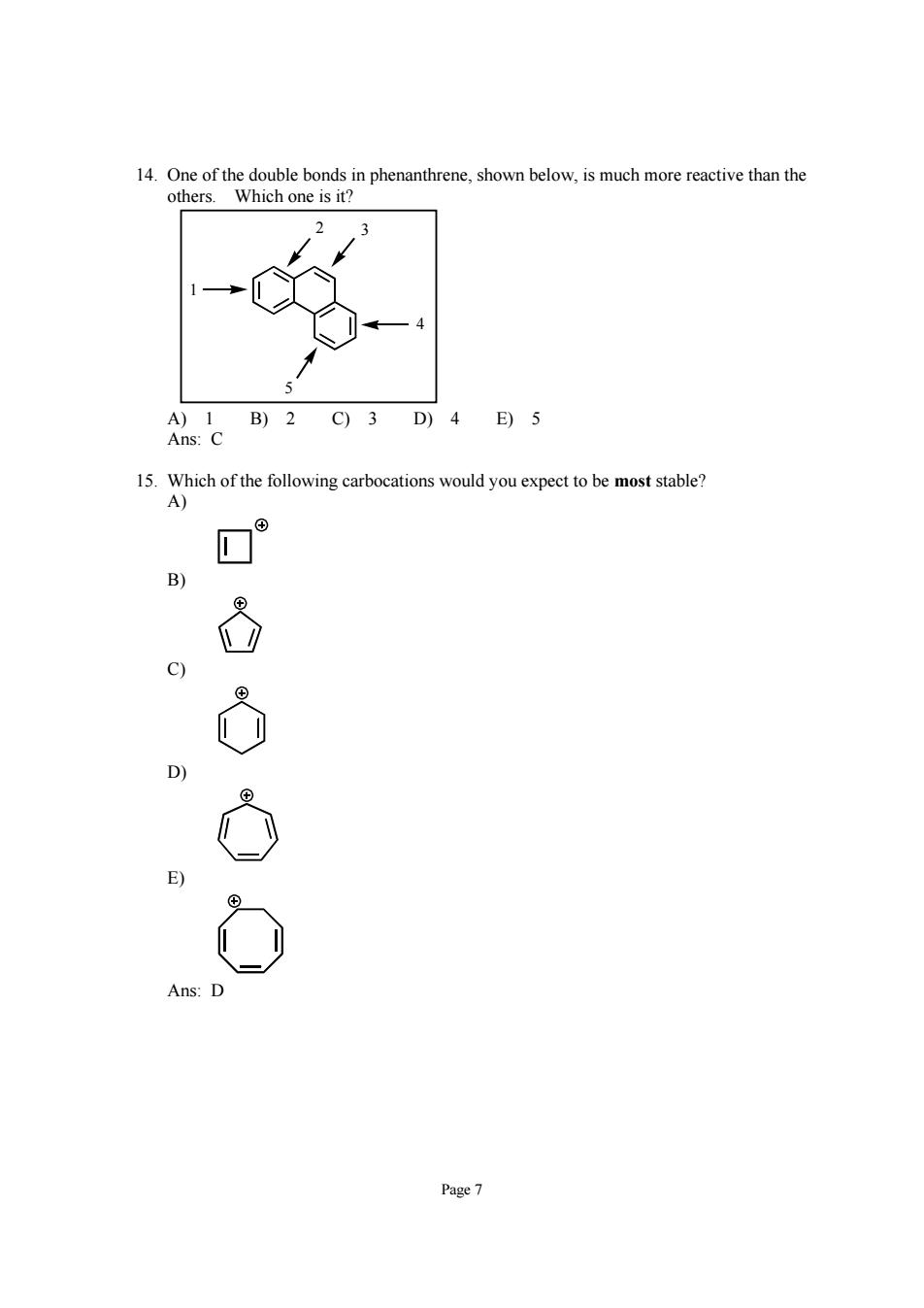
others.Which one is it? B)2 C)3D)4 E)5 15.Which of the following carbocations would you expect to be most stable? Ans Page7
Page 7 14. One of the double bonds in phenanthrene, shown below, is much more reactive than the others. Which one is it? 5 4 2 3 1 A) 1 B) 2 C) 3 D) 4 E) 5 Ans: C 15. Which of the following carbocations would you expect to be most stable? A) B) C) D) E) Ans: D

16.Which of the following molecules would you predict to be aromatic? A) C Ans:B OH OCH; vanillin CHO }场m& 3-formyl-6-hydroxvanisole imt好meoy phen 4-formyl-5-methoxyphenol C)4-hydroxy-3-methoxybenzaldehyde Ans:C Page8
Page 8 16. Which of the following molecules would you predict to be aromatic? A) B) O C) D) E) H Ans: B 17. Which would be the best systematic name of vanillan, the primary flavoring ingredient in vanilla? OH CHO OCH3 vanillin A) 4-formyl-2-methoxyphenol D) 5-formyl-2-hydroxyanisole B) 3-formyl-6-hydroxyanisole E) 4-formyl-5-methoxyphenol C) 4-hydroxy-3-methoxybenzaldehyde Ans: C
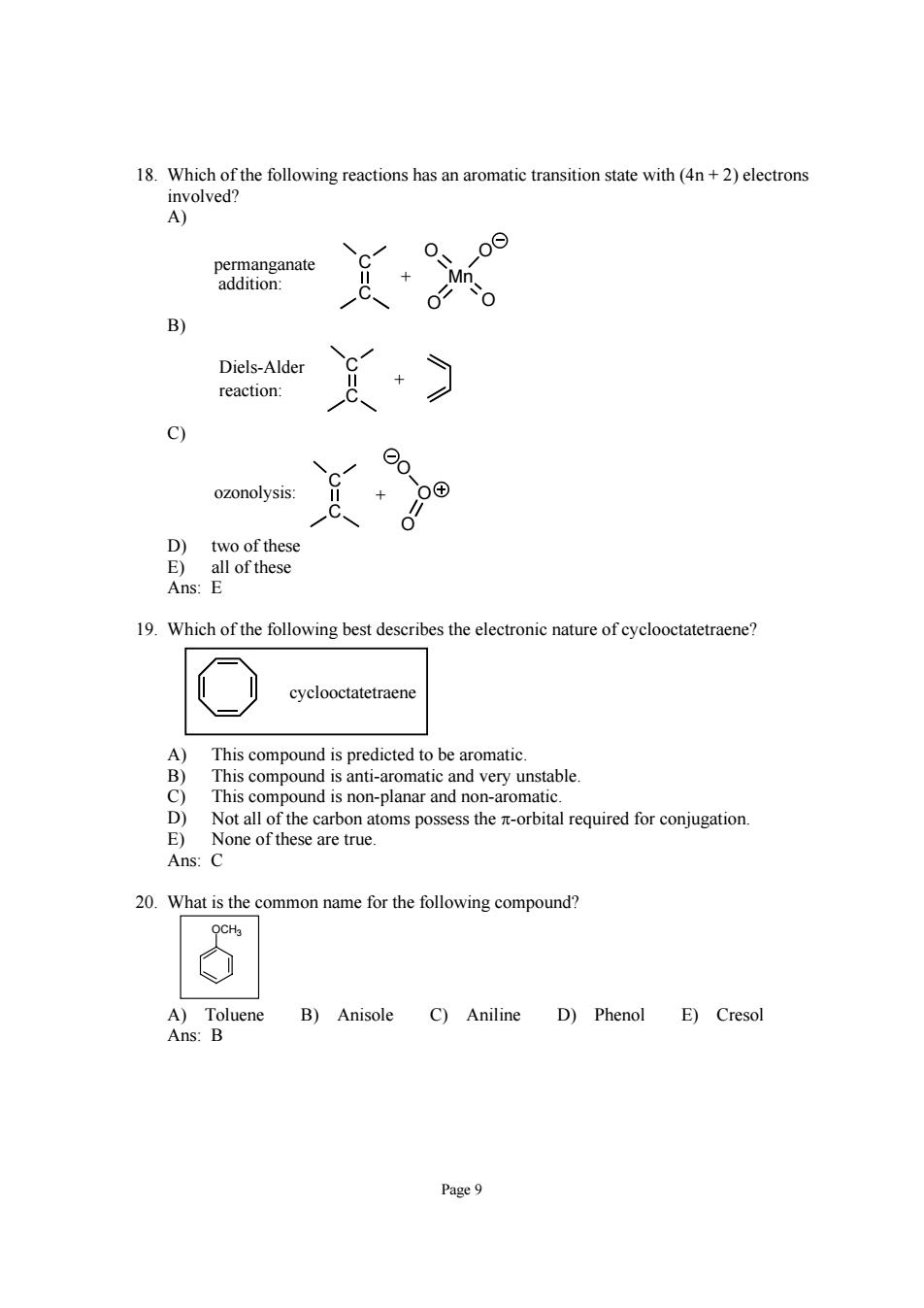
黑cah0 pGdaeanaie Diels-Alder reaction: 9 ozonolysis D)two of these E)all of these Ans:E 19.Which of the following best describes the electronic nature of cyclooctatetraene This compound is predicted to be aromatic. This compound is anti-aromatic and very unstable. This compound is non-planar and non-aromatic Not all the carbon atoms possess the -orbital required for conjugation. 20.What is the common name for the following compound? A)Toluene B)Anisole C)Aniline D)Phenol E)Cresol Ans:B Page9
Page 9 18. Which of the following reactions has an aromatic transition state with (4n + 2) electrons involved? A) C C O Mn O O O + permanganate addition: B) C C + Diels-Alder reaction: C) C C O O O ozonolysis: + D) two of these E) all of these Ans: E 19. Which of the following best describes the electronic nature of cyclooctatetraene? cyclooctatetraene A) This compound is predicted to be aromatic. B) This compound is anti-aromatic and very unstable. C) This compound is non-planar and non-aromatic. D) Not all of the carbon atoms possess the π-orbital required for conjugation. E) None of these are true. Ans: C 20. What is the common name for the following compound? OCH3 A) Toluene B) Anisole C) Aniline D) Phenol E) Cresol Ans: B
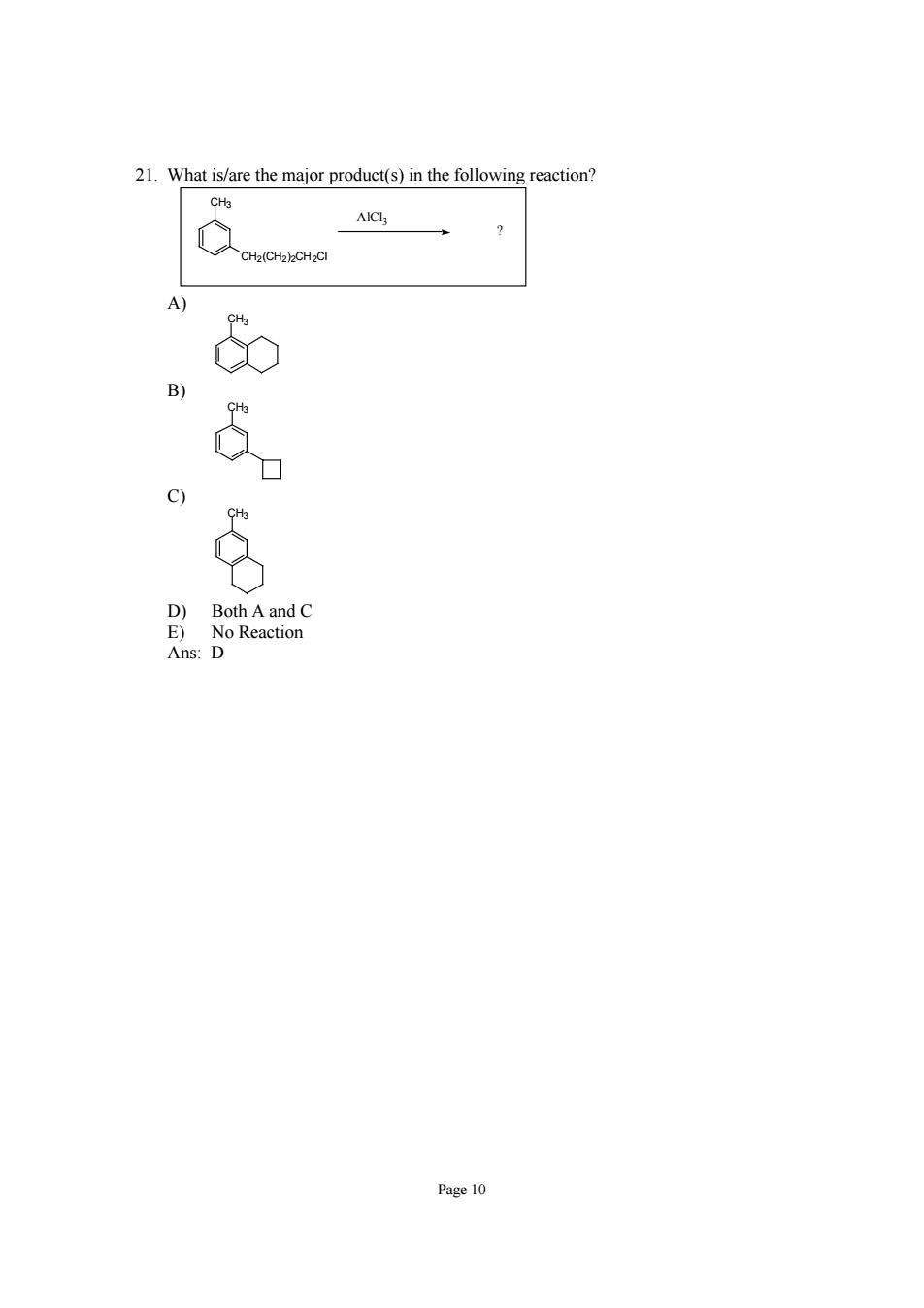
21.What is/are the major product(s)in the following reaction? CH2( o 9 8 D)Both A and C E)No Reaction Ans:D Page 10
Page 10 21. What is/are the major product(s) in the following reaction? CH3 AlCl3 CH2(CH2)2CH2Cl ? A) CH3 B) CH3 C) CH3 D) Both A and C E) No Reaction Ans: D