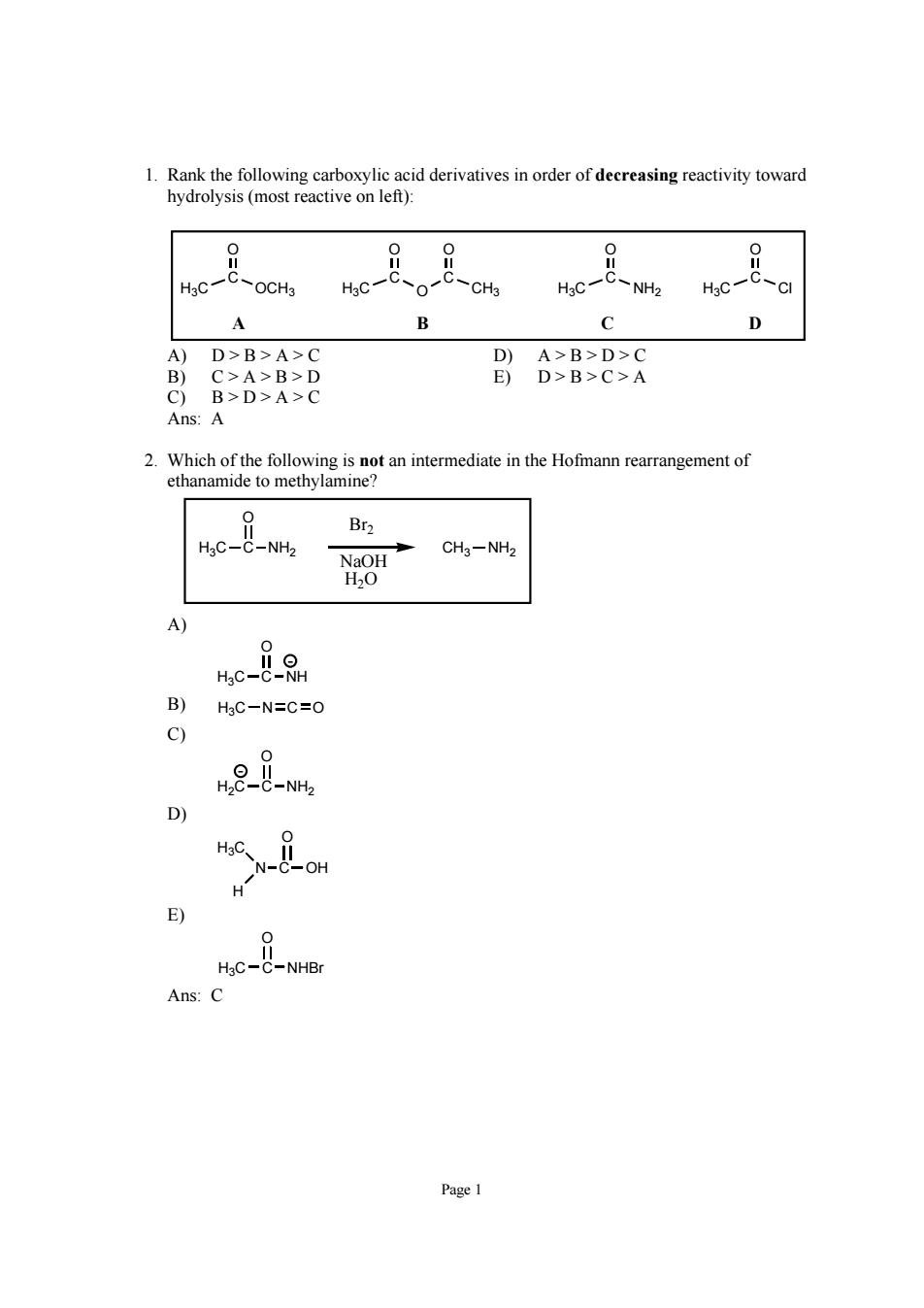
1.Rank the following carboxylic acid derivatives in order of decreasing reactivity toward hydrolysis(most reactive on left): 88 8 OCH3 HaC NH2 CI A B D A)D>B>A>C D)A>B>D>C B)C>A>B>D E)D>B>C>A C)B>D>A>C Ans:A Br2 8 CH3-NH hc-88 B) HgC-N=C=O n2- E) e8-Ne Ans:C
Page 1 1. Rank the following carboxylic acid derivatives in order of decreasing reactivity toward hydrolysis (most reactive on left): H3C C O H3C Cl C O OCH3 H3C C O H3C NH2 C O O CH3 C O A B CD A) D > B > A > C D) A > B > D > C B) C > A > B > D E) D > B > C > A C) B > D > A > C Ans: A 2. Which of the following is not an intermediate in the Hofmann rearrangement of ethanamide to methylamine? H3C C O NH2 CH3 NH2 Br2 NaOH H2O A) H3C C O NH - B) H3CN O C C) H2C C O NH2 - D) O H OH H3C N C E) H3C C O NHBr Ans: C

3.In the reaction shown below.which product(s)would be formed? H;C-CH2-C -OH CH,"OH HgC-CH2- HgC-CH2-c -OCH3 C) 16 HC-CH-CoH Page2
Page 2 3. In the reaction shown below, which product(s) would be formed? H3C CH2 C O OH ? H+ heat + CH3 18OH A) H3C CH2 C O OCH3 18 B) H3C CH2 C O OCH3 18 C) H3C CH2 C O OCH3 18 18 D) H3C CH2 C O OH 16 16 E) both A and B Ans: A
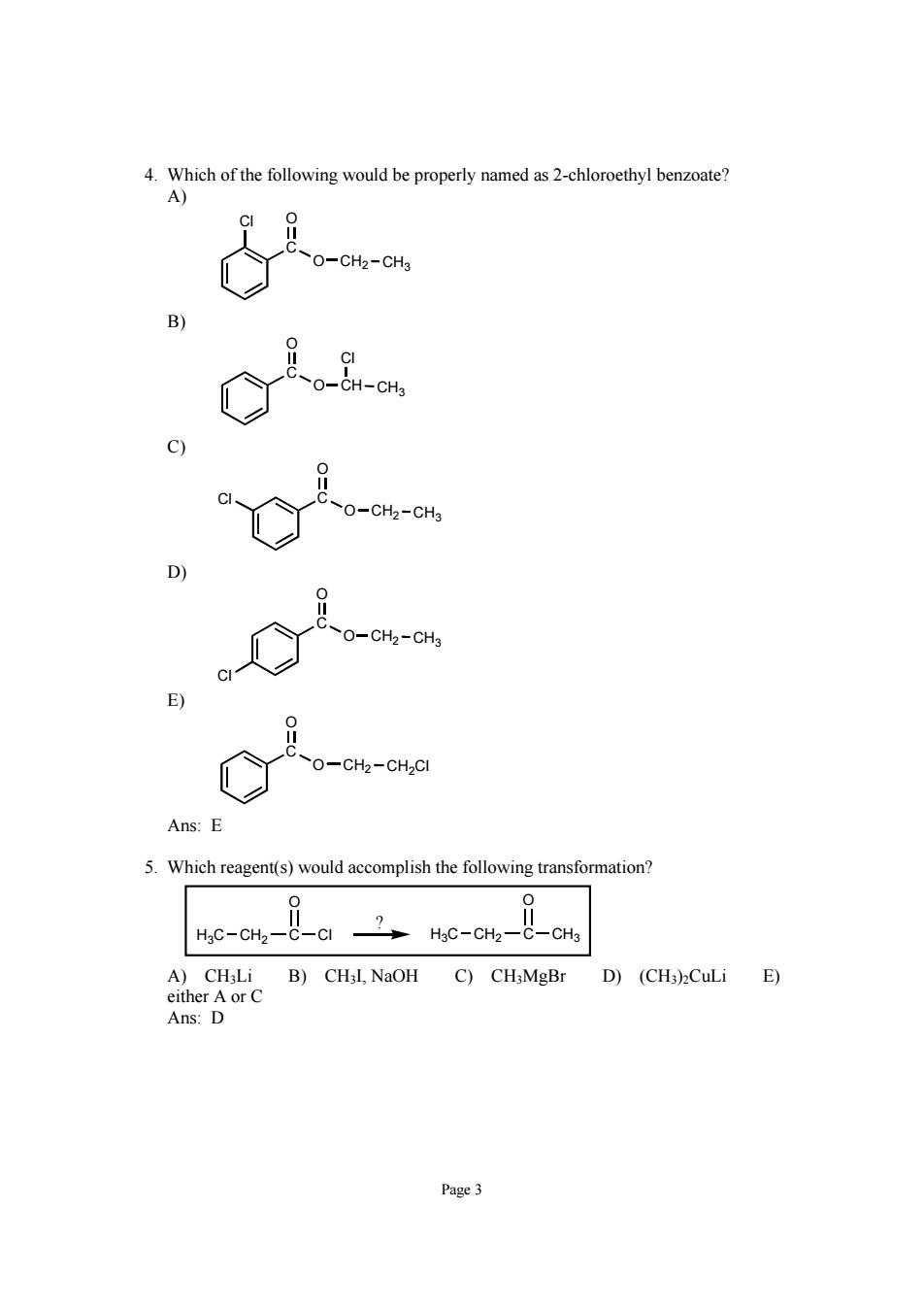
4.Which of the following would be properly named as 2-chloroethyl benzoate? A) O-CH2-CHa O-CH2-CHa O-CHz-CHa O-CHz-CH2CI Ans:E 5.Which reagent(s)would accomplish the following transformation? A)CH;Li B)CHL,NaOH C)CH;MgBr D)(CHa)2CuLi E) either A or C Ans:D Page 3
Page 3 4. Which of the following would be properly named as 2-chloroethyl benzoate? A) CH2 CH3 C O O Cl B) CH CH3 C O O Cl C) CH2 CH3 C O O Cl D) CH2 CH3 C O O Cl E) CH2 CH2Cl C O O Ans: E 5. Which reagent(s) would accomplish the following transformation? H3C CH2 C O Cl H3C CH2 C O CH3 ? A) CH3Li B) CH3I, NaOH C) CH3MgBr D) (CH3)2CuLi E) either A or C Ans: D

HC-C-N(CH2 A)One B)Two C)Three D)Four E)Five Ans:C 7.What would be the name of the following cyclic ester? 1 A)y-valerolactone D)2-methyl-B-butyrolactone B)B-butyrolactone E)2-methyl-y-valerolactone C)2-methyl-y-butyrolactone Ans:C .Whatouor the fact that the moecbewracemies easily espite having three chiral centers HOH.C actones are configurati onally unstable MoleceulelIosesCoet E Intra fication occurs easily This molecule is not chiral. s:D Page4
Page 4 6. How many different CH3 signals would you expect in the room-temperature proton NMR spectrum of the molecule below? H3C C O N(CH3)2 A) One B) Two C) Three D) Four E) Five Ans: C 7. What would be the name of the following cyclic ester? O O H3C A) γ-valerolactone D) 2-methyl-β-butyrolactone B) β-butyrolactone E) 2-methyl-γ-valerolactone C) 2-methyl-γ-butyrolactone Ans: C 8. What would account for the fact that the molecule below racemizes easily, despite having three chiral centers? O O H H HOH2C A) Lactones are configurationally unstable B) Molecule loses CO2 easily C) Enolization occurs easily D) Intramolecular transesterification occurs easily E) This molecule is not chiral. Ans: D
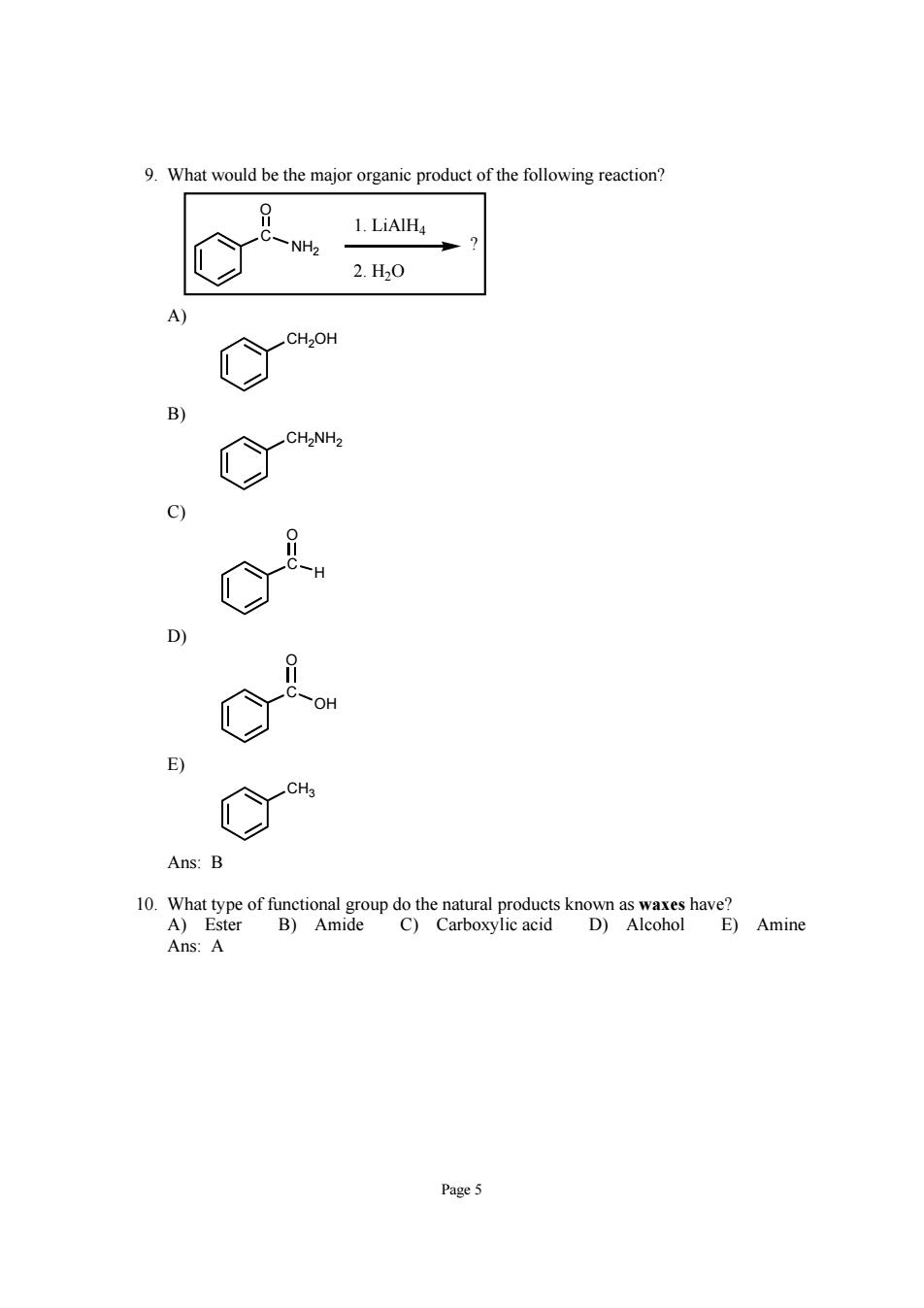
9.What would be the major organic product of the following reaction? 0 1.LiAlH4 -NH2 2.H0 3 C Ans:B Ans:A Page5
Page 5 9. What would be the major organic product of the following reaction? C O NH2 ? 1. LiAlH4 2. H2O A) CH2OH B) CH2NH2 C) C O H D) C O OH E) CH3 Ans: B 10. What type of functional group do the natural products known as waxes have? A) Ester B) Amide C) Carboxylic acid D) Alcohol E) Amine Ans: A
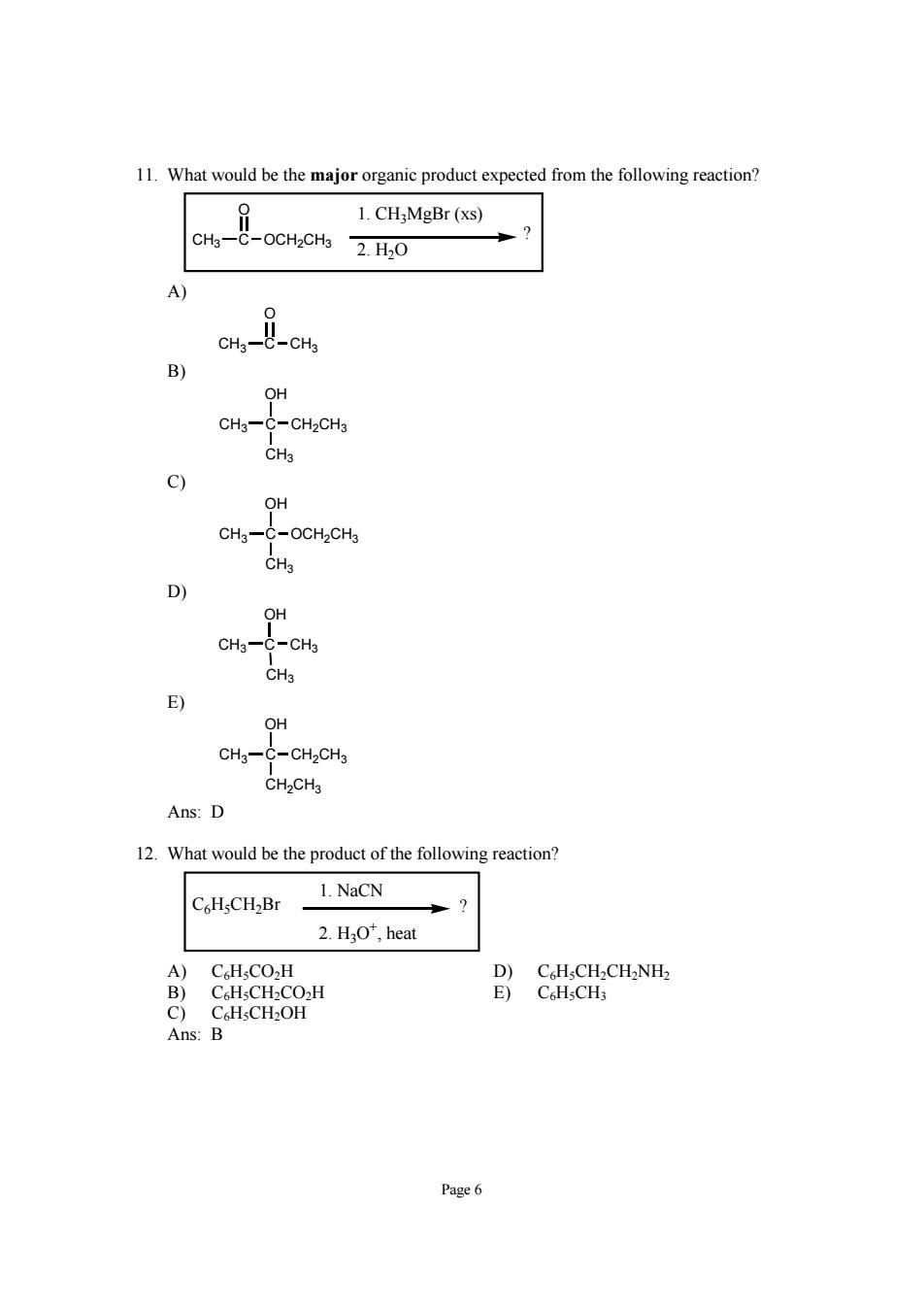
11.What would be the major organic product expected from the following reaction? 1.CHMgBr (xs) CH3- 2.H0 Cn--cm OH CH3- -CH2CHs OH cH3一 -OCH2CH3 D CH3- -CH3 CH3 CH3- -CH2CH3 CH.CHs Ans:D 12.What would be the product of the following reaction? 1.NaCN 2.HO",heat B)CH-CH-CO-H C)CsHsCH2OH Ans:B Page6
Page 6 11. What would be the major organic product expected from the following reaction? CH3 C O OCH2CH3 ? 1. CH3MgBr (xs) 2. H2O A) CH3 C O CH3 B) CH3 C OH CH2CH3 CH3 C) CH3 C OH OCH2CH3 CH3 D) CH3 C CH3 CH3 OH E) CH3 C OH CH2CH3 CH2CH3 Ans: D 12. What would be the product of the following reaction? C6H5CH2Br ? 1. NaCN 2. H3O+, heat A) C6H5CO2H D) C6H5CH2CH2NH2 B) C6H5CH2CO2H E) C6H5CH3 C) C6H5CH2OH Ans: B
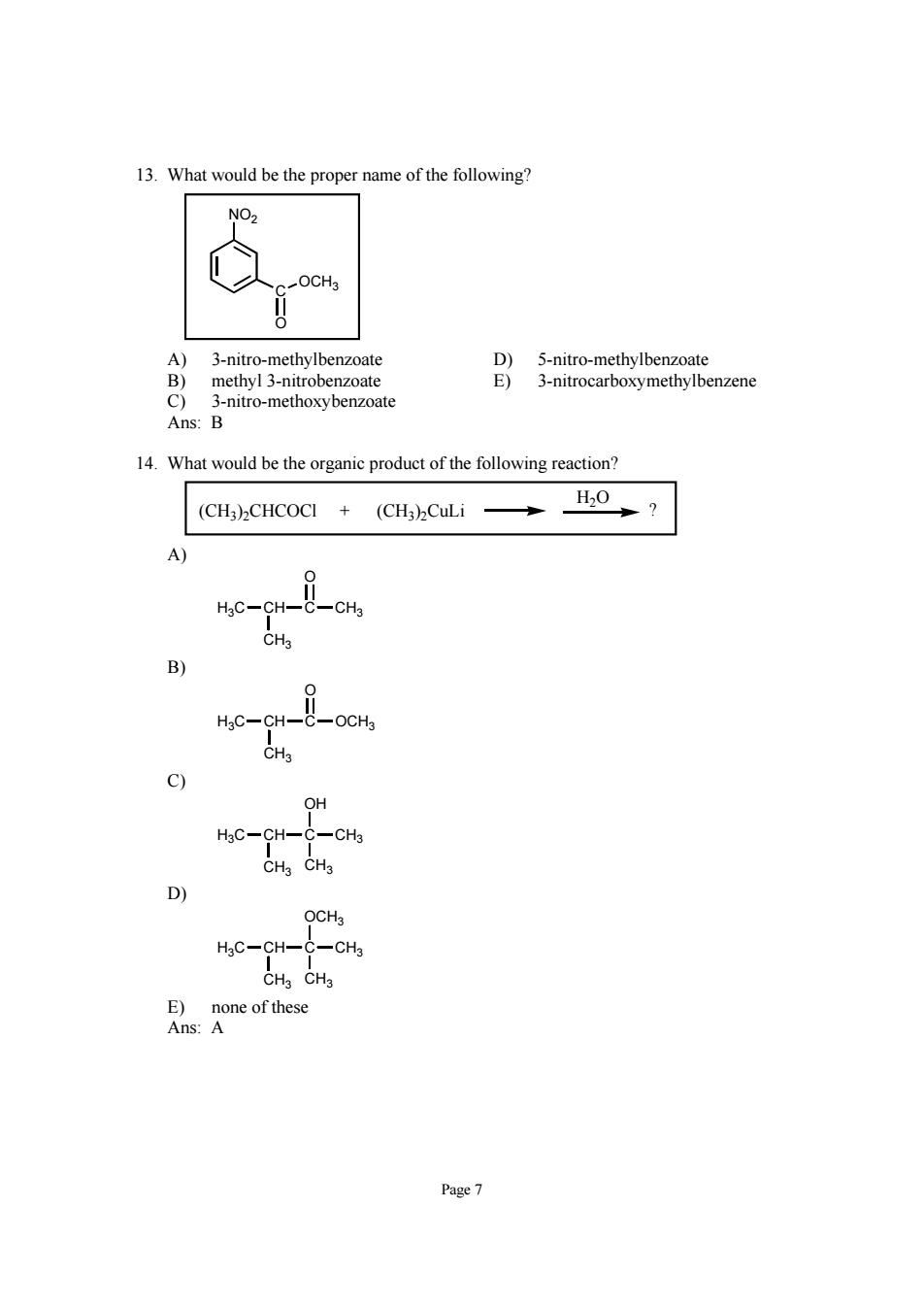
13.What would be the proper name of the following? NO2 A) 3-nitro 5-nitro-methylbenzoate 3-nitre c B-nitro-methoxybenzoate Ans:B 14.What would be the organic product of the following reaction? (CH3)CHCOCI HgC-CH- CH. HgC-CH- OH HgC-CH- -CH3 CHa CH3 D OCH3 HgC-CH-C-CH3 CHa CHa E)none of these Ans: Page7
Page 7 13. What would be the proper name of the following? NO2 C O OCH3 A) 3-nitro-methylbenzoate D) 5-nitro-methylbenzoate B) methyl 3-nitrobenzoate E) 3-nitrocarboxymethylbenzene C) 3-nitro-methoxybenzoate Ans: B 14. What would be the organic product of the following reaction? (CH3)2CHCOCl + (CH3)2CuLi H2O ? A) H3C CH CH3 C O CH3 B) H3C CH CH3 C O OCH3 C) H3C CH CH3 C OH CH3 CH3 D) H3C CH CH3 C OCH3 CH3 CH3 E) none of these Ans: A
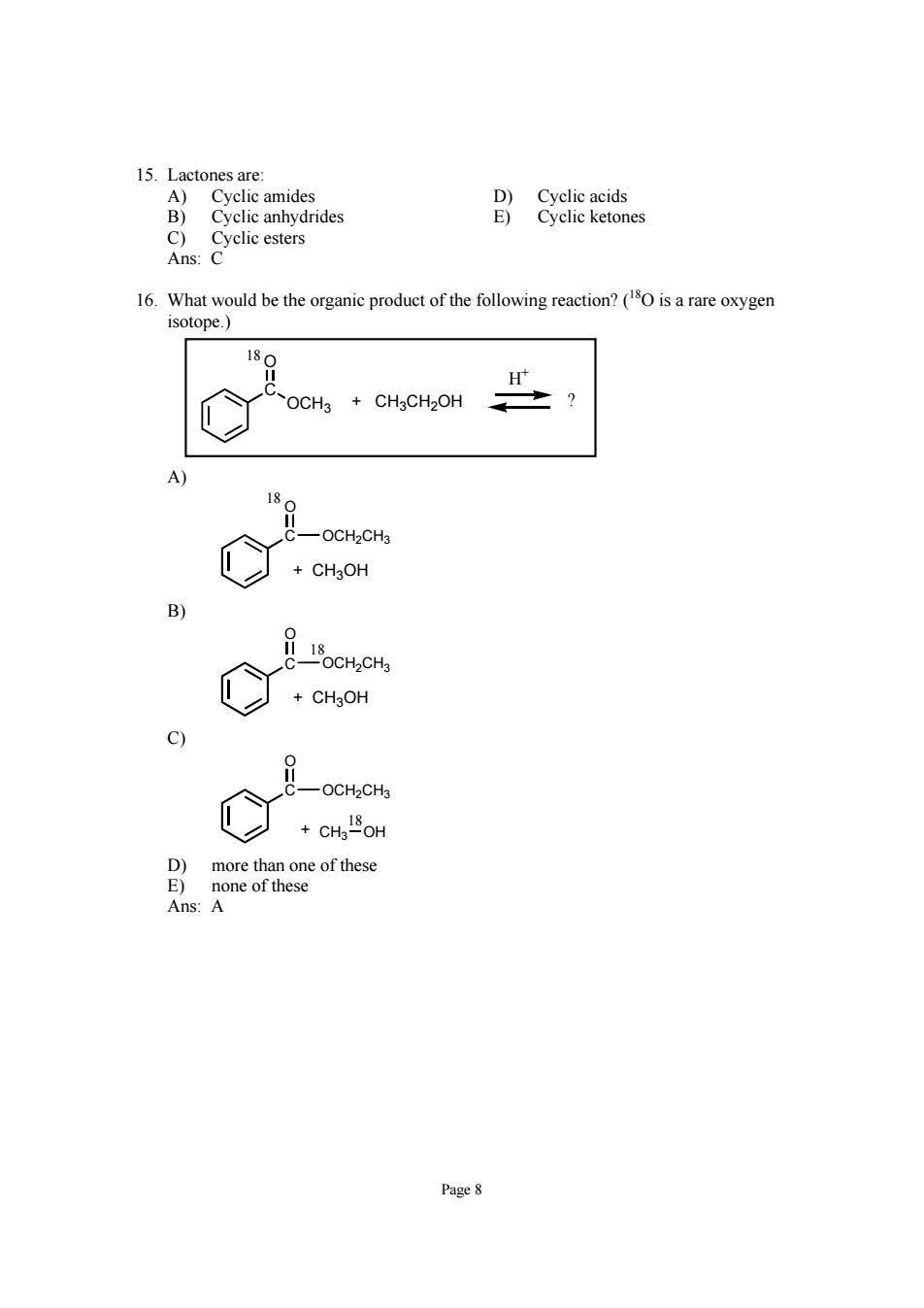
15.Lactones are 16.What would be the organic product of the following reaction?(is a rare oxygen isotope.) OCH CHgCH2OH 18 一OCH2CH3 B) c) &一OCH-CH D)more than one of these none of these Ans Page 8
Page 8 15. Lactones are: A) Cyclic amides D) Cyclic acids B) Cyclic anhydrides E) Cyclic ketones C) Cyclic esters Ans: C 16. What would be the organic product of the following reaction? (18O is a rare oxygen isotope.) C OCH3 O ? H+ + CH3CH2OH 18 A) C OCH2CH3 O + CH3OH 18 B) C OCH2CH3 O + CH3OH 18 C) C OCH2CH3 O 18 + CH3 OH D) more than one of these E) none of these Ans: A

17.What would be the organic product of the following reaction? (-)-mentho )g ornd E)no reaction occurs Ans:A Page9
Page 9 17. What would be the organic product of the following reaction? CH3 OH OH O (-)-menthol + H+ ? A) CH3 O O B) CH3 O O C) mixture of A and B D) none of these E) no reaction occurs Ans: A
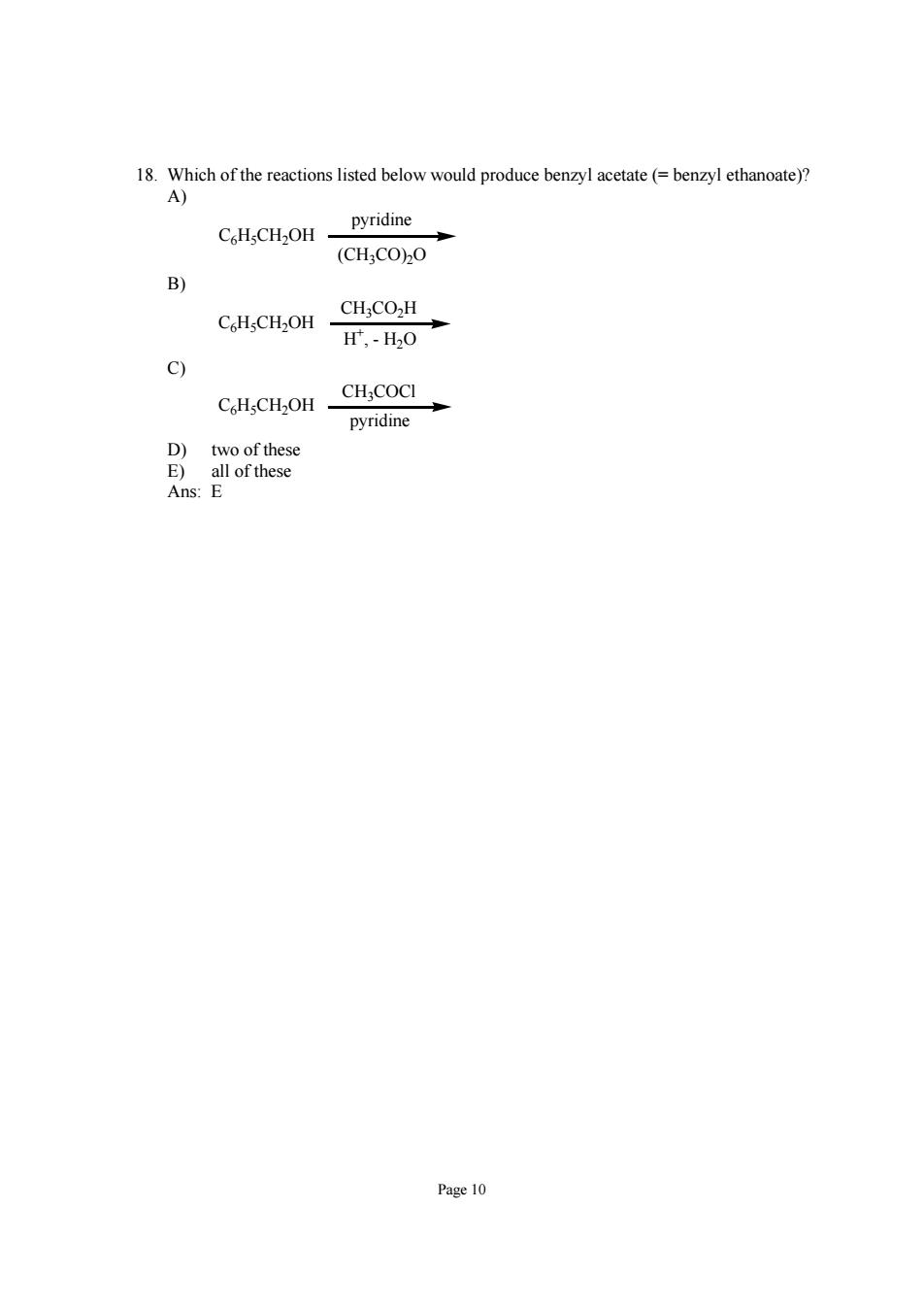
1.Which produce benzy (benzy ethanoate)? pyridine C.HsCH2OH (CH:COO B) CHsCH2OH 0 9 CH:COCI pyridine E) Ans:E Page 10
Page 10 18. Which of the reactions listed below would produce benzyl acetate (= benzyl ethanoate)? A) (CH3CO)2O C6H5CH2OH pyridine B) CH3CO2H C6H5CH2OH H+ , - H2O C) CH3COCl C6H5CH2OH pyridine D) two of these E) all of these Ans: E