1sceokarmhoimnaec时rokopoundnoaga A)C1oHis B)C1oH20 C)C1oHi6 D)CnH4 E)CuHis Ans:C 2.Of those indicated,which would be the shortest carbon-carbon bond in a-selinene? A)A B)B C)C D)D E)E Ans:B What would be the ideal value for the indicated bond angle? C)104° D)180°E)109
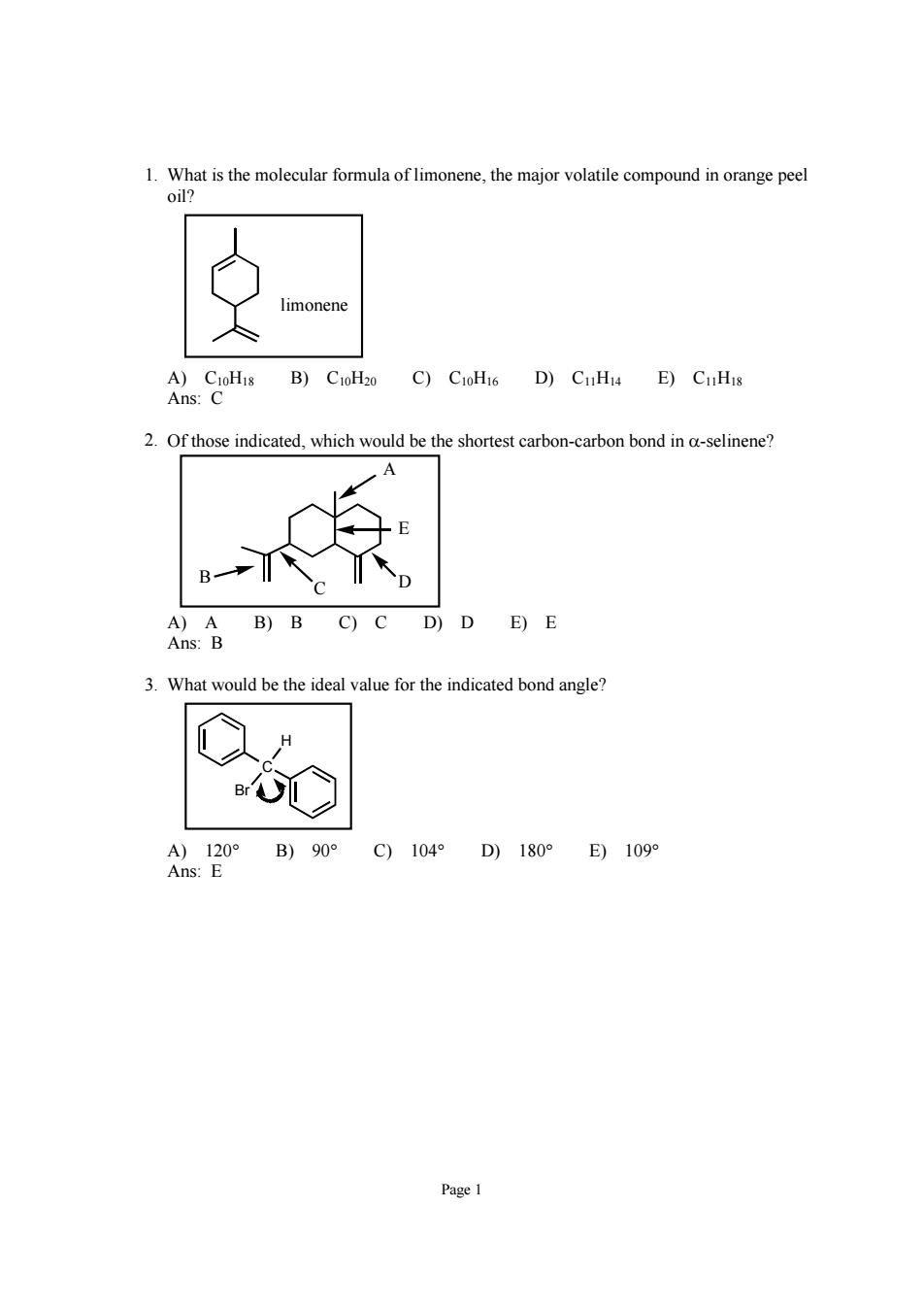
Page 1 1. What is the molecular formula of limonene, the major volatile compound in orange peel oil? limonene A) C10H18 B) C10H20 C) C10H16 D) C11H14 E) C11H18 Ans: C 2. Of those indicated, which would be the shortest carbon-carbon bond in α-selinene? E D C B A A) A B) B C) C D) D E) E Ans: B 3. What would be the ideal value for the indicated bond angle? C H Br A) 120° B) 90° C) 104° D) 180° E) 109° Ans: E
What would be the"polygon"form of the following condensed structure CHCH2CH(CN)CH=CHz Page2
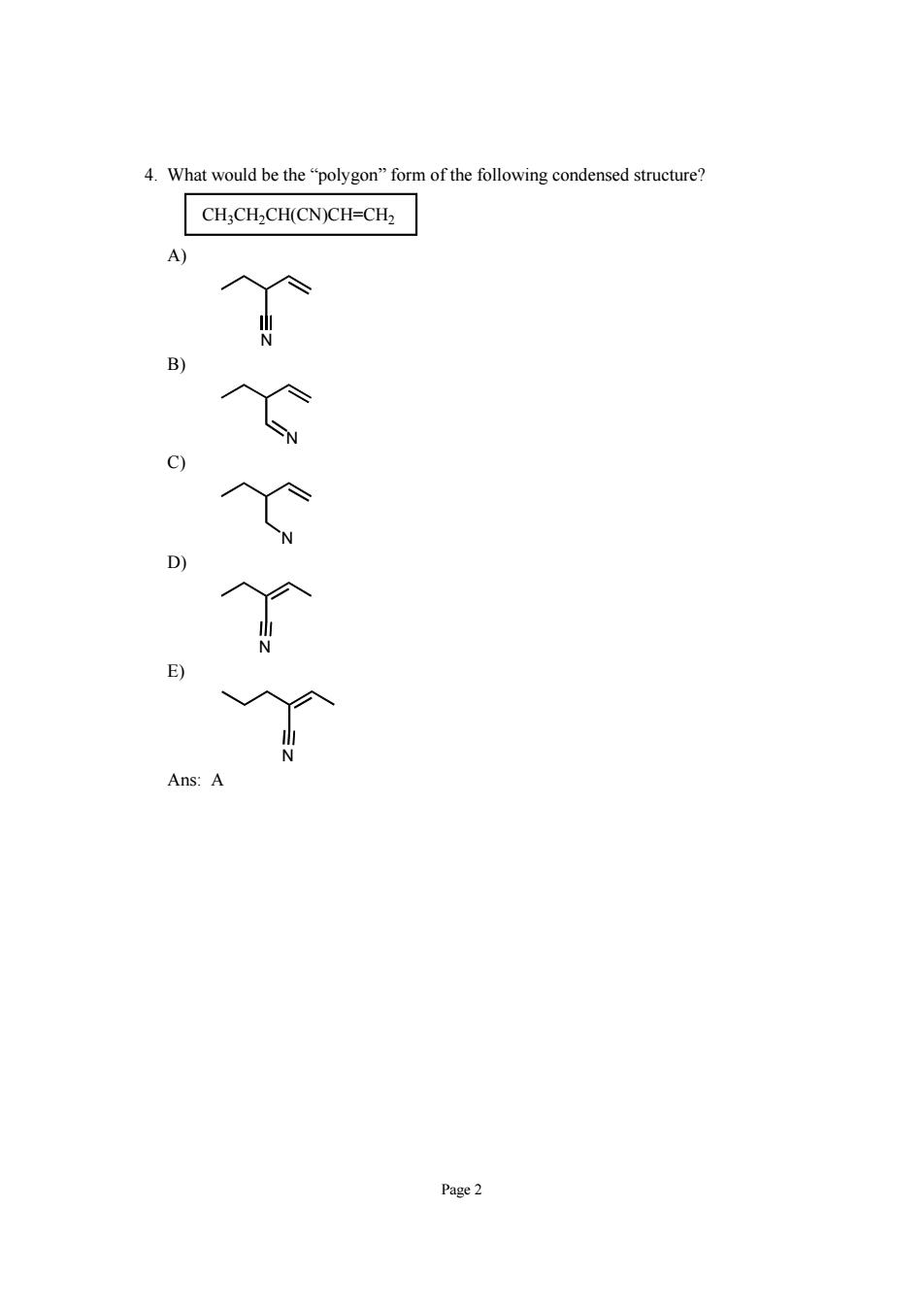
Page 2 4. What would be the “polygon” form of the following condensed structure? CH3CH2CH(CN)CH=CH2 A) N B) N C) N D) N E) N Ans: A

.Whch of h following structures must nore 0以0 Page
Page 3 5. Which one of the following structures must be incorrect? A) OH B) O C) O D) C C E) Ans: C
not a resonance structure of the others? Page 4
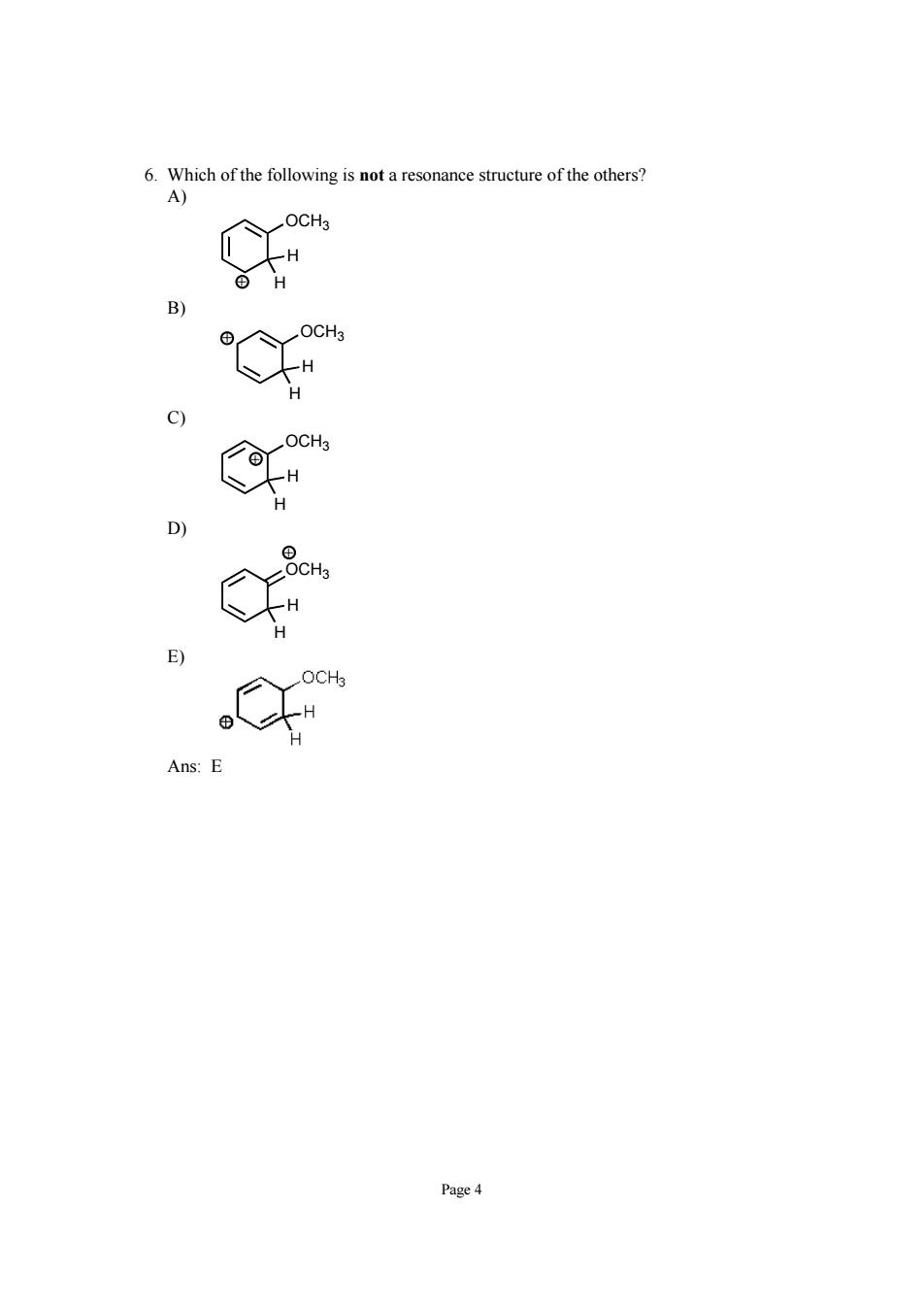
Page 4 6. Which of the following is not a resonance structure of the others? A) OCH3 H H + B) OCH3 H H + C) OCH3 H H + D) OCH3 H H + E) Ans: E
Which one of the resonance structures below would be the most important(ie..most How many atoms in ethene are required by spbonding to lie in the same plane? c=c ethene 0234D)56 Page 5
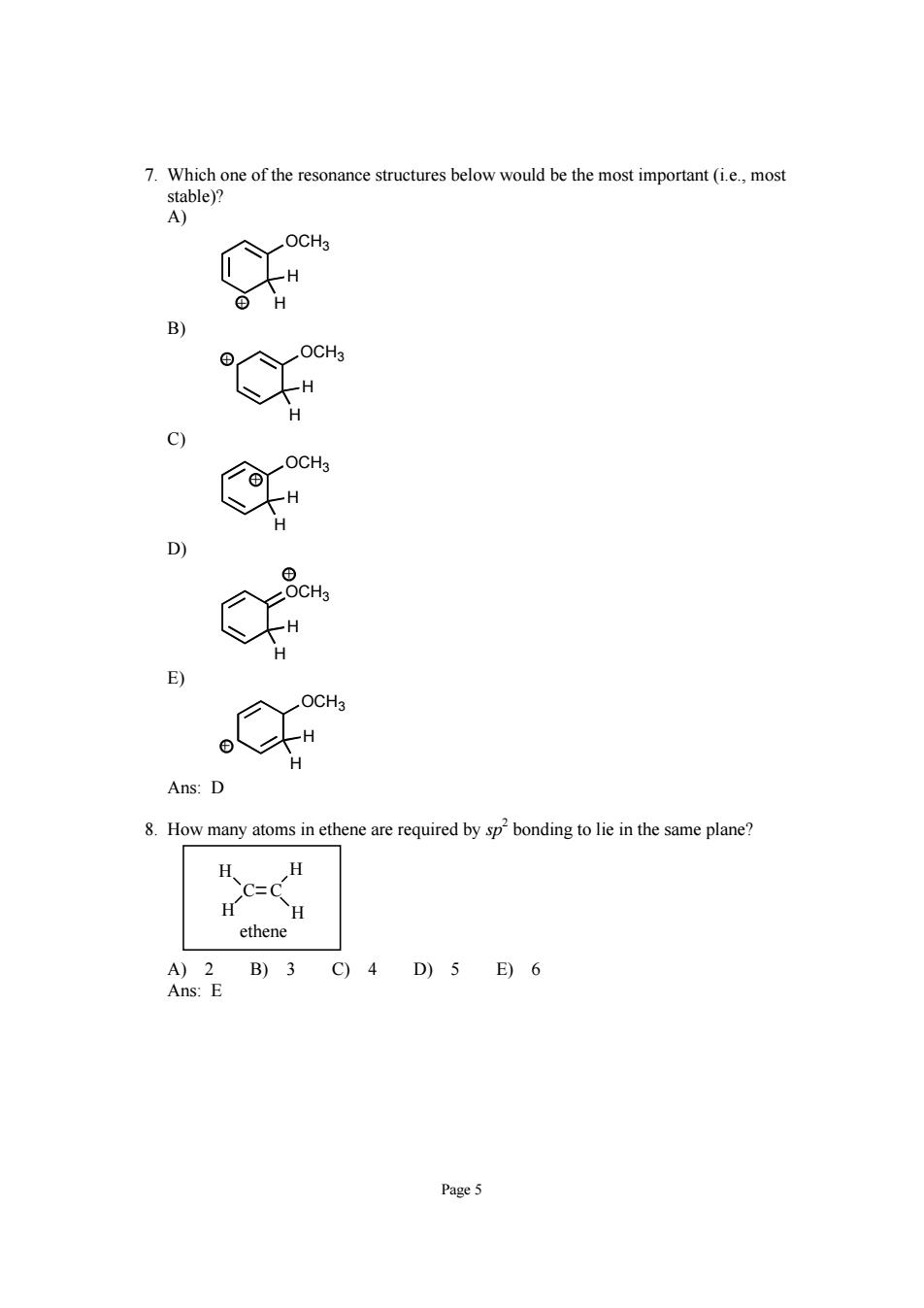
Page 5 7. Which one of the resonance structures below would be the most important (i.e., most stable)? A) OCH3 H H + B) OCH3 H H + C) OCH3 H H + D) OCH3 H H + E) OCH3 H H + Ans: D 8. How many atoms in ethene are required by sp 2 bonding to lie in the same plane? C C H H H H ethene A) 2 B) 3 C) 4 D) 5 E) 6 Ans: E

9.Which one of the following structures is not chemically identical to the others? HgC-CH2 CH2-CH3 H2C- -CH CHa CH3-CH2 H2C CH3-CH2-CH-CH3 CH3 HgC-CH2 CH2 H3C-CCH2 D) H3C-CH2 CHs H3C-0 -CH2-CH3 夕 CH2-CH3 HC-CH3 CH3-CH2-CH2 Ans:D Page6
Page 6 9. Which one of the following structures is not chemically identical to the others? A) H3C CH2 H2C CH CH2 CH3 CH3 B) CH3 CH2 H2C CH3 CH2 CH CH3 C) H3C H3C CH2 C CH2 CH2 CH3 H D) H3C H3C CH2 C CH2 CH3 CH3 H E) CH2 CH3 HC CH3 CH2 CH2 CH3 Ans: D

10.Which of the following pairs are not resonance structures of each other? A) H-CEC-H d MC=C=CH B) a and合 11.How many hydrogen atoms are part of the following steroid? OH A)18B)20C)21D)22E)24 Ans:E 2.In the following molecule,how many carbon atoms are in the sphybridization state 〔文 OCH A)2B)4C)5D)6E)11 Ans:D Page7
Page 7 10. Which of the following pairs are not resonance structures of each other? A) H2CCCH H and 2C C CH - - B) O O - - and C) C NH2 O C NH2 O- + and D) C CH2 O C CH2 O - - and E) All are pairs of resonance structures Ans: E 11. How many hydrogen atoms are part of the following steroid? O OH A) 18 B) 20 C) 21 D) 22 E) 24 Ans: E 12. In the following molecule, how many carbon atoms are in the sp 3 hybridization state? OCH3 O A) 2 B) 4 C) 5 D) 6 E) 11 Ans: D
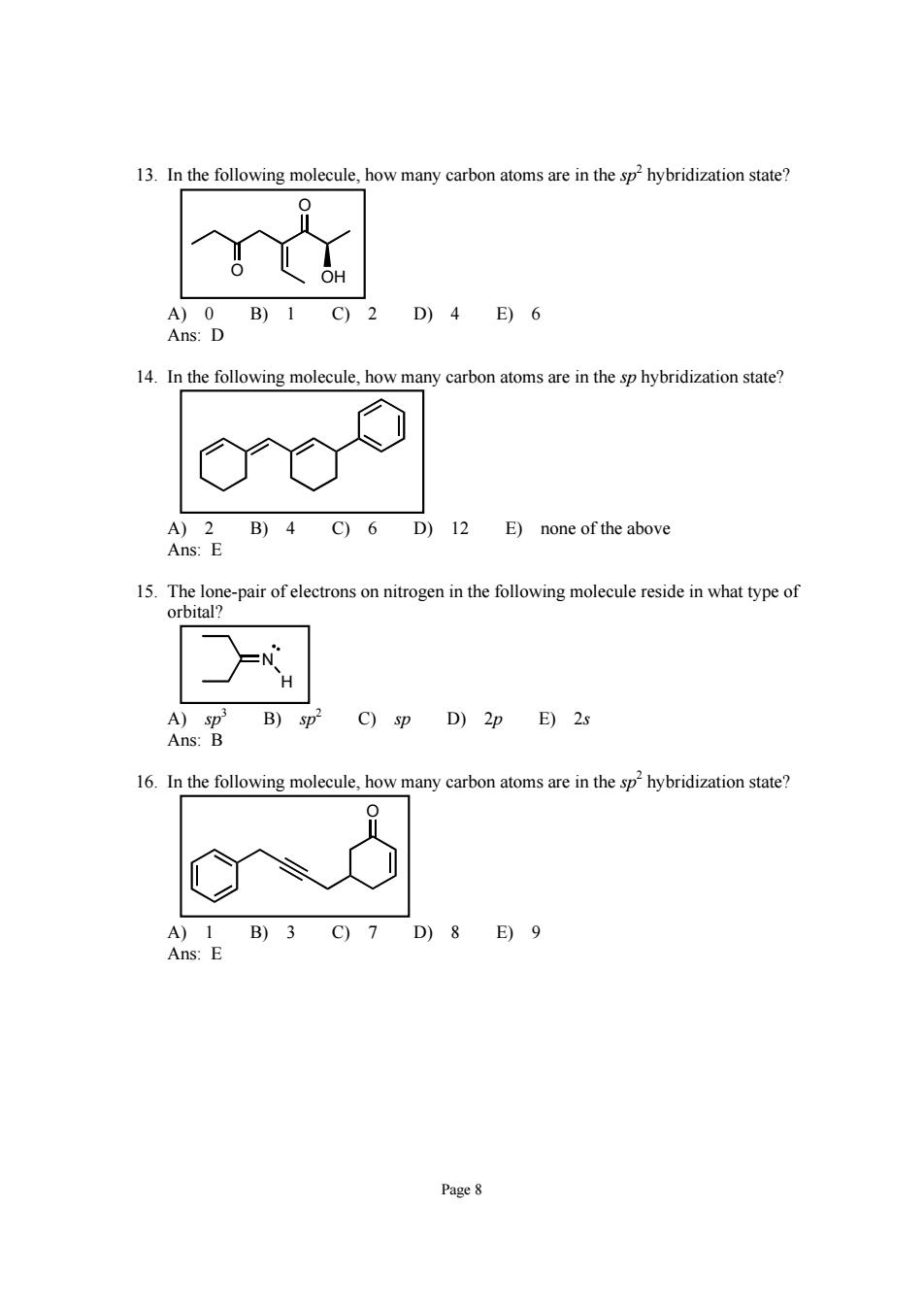
13.In the following molecule,how many carbon atoms are in the sp2hybridization state? A)0B)1C)2D)4E)6 Ans:D 14.In the following molecule,how many carbon atoms are in the sp hybridization state? B)4C)6 D)12 E)none of the above 15.The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? C)sp D)2p E)2s 16.In the following molecule how many carbon atoms are in the sphybridization state A)1B)3C)7D)8E)9 Ans:E Page 8
Page 8 13. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O O OH A) 0 B) 1 C) 2 D) 4 E) 6 Ans: D 14. In the following molecule, how many carbon atoms are in the sp hybridization state? A) 2 B) 4 C) 6 D) 12 E) none of the above Ans: E 15. The lone-pair of electrons on nitrogen in the following molecule reside in what type of orbital? H N A) sp 3 B) sp 2 C) sp D) 2p E) 2s Ans: B 16. In the following molecule, how many carbon atoms are in the sp 2 hybridization state? O A) 1 B) 3 C) 7 D) 8 E) 9 Ans: E

17.The boxed item most likely represents what? none of the above Ans 18.The following molecule contains how many carbon atoms in the sp hybridization state? Ais:A B)3 C)8D)13E)16 19.The nitrogen A) methyineCHNconshor B)one c) molecule two D)three Ans:B 20.A positive charge on oxygen generally occurs when: oxygen has too many electrons. oxygen has too few electrons. onding pairs 21.The carbon atom in CH2Cl2 has what hybridization? C)sp'D)sp*E)they are not hybridized 22.The molecular formula for piperitone is piperitone A)CHi6O B)C1oHisO C)C.HisO D)C10H4O E)C10H16O Ans:E Page9
Page 9 17. The boxed item most likely represents what? A) s orbital D) could be any of A–C B) sp 3 orbital E) none of the above C) p orbital Ans: B 18. The following molecule contains how many carbon atoms in the sp hybridization state? C O A) 1 B) 3 C) 8 D) 13 E) 16 Ans: A 19. The nitrogen of trimethylamine [(CH3)3N] contains how many lone pairs of electrons? A) none B) one C) two D) three E) there is no nitrogen in this molecule Ans: B 20. A positive charge on oxygen generally occurs when: A) oxygen has too many electrons. B) oxygen has too few electrons. C) oxygen is sharing one of its non-bonding electron pairs. D) oxygen has too many non-bonding electron pairs. E) oxygen is borrowing electrons from another atom. Ans: C 21. The carbon atom in CH2Cl2 has what hybridization? A) sp B) sp 2 C) sp 3 D) sp 4 E) they are not hybridized Ans: C 22. The molecular formula for piperitone is O piperitone A) C9H16O B) C10H18O C) C9H18O D) C10H14O E) C10H16O Ans: E
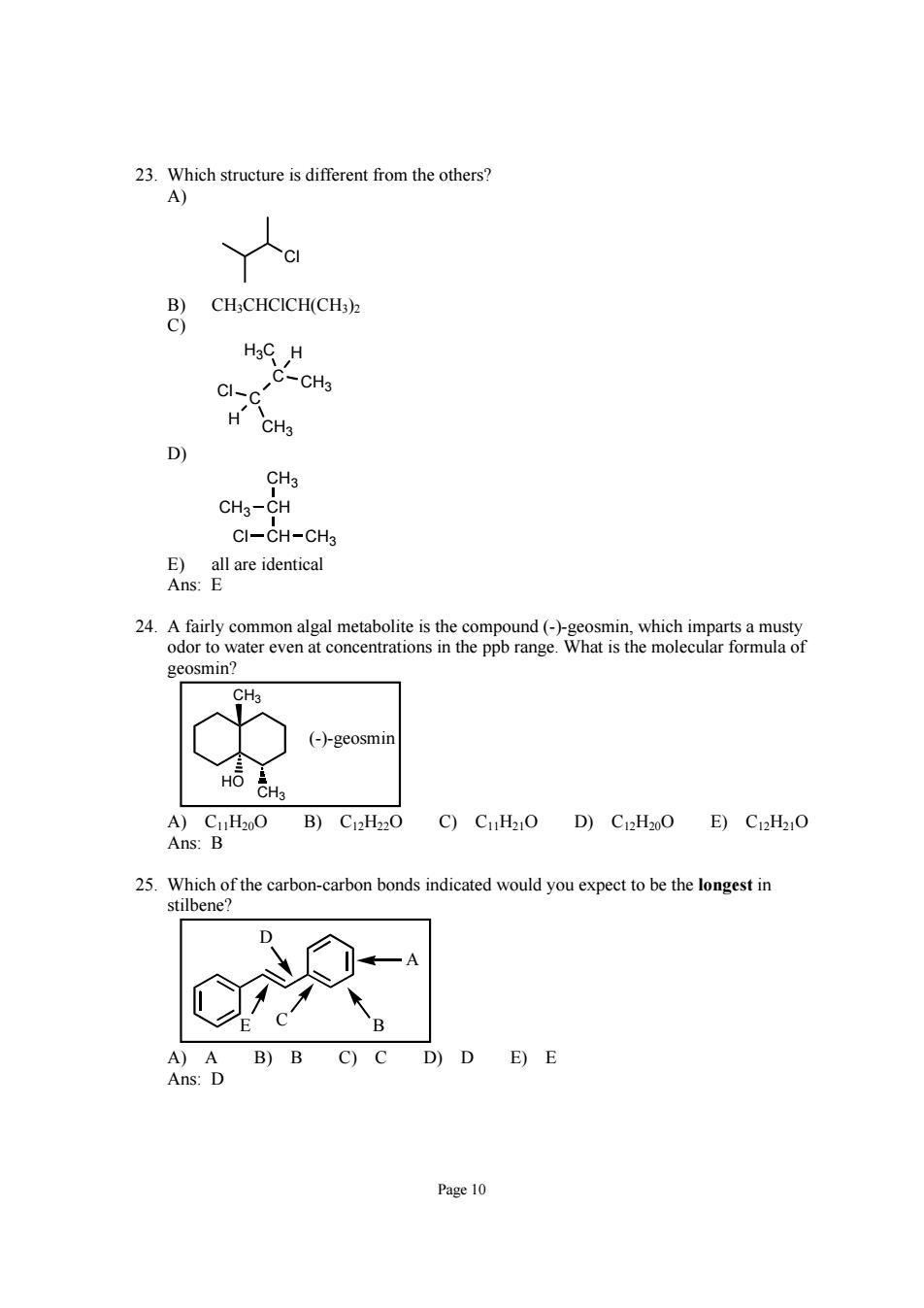
23.Which structure is different from the others? CHCHCICHICH C-CH3 D 3 CH5-CH CI-CH-CH3 及alre enteal geosmin? (-)-geosmi A)C1H200B)C12H220 C)CuH21O D)C12H20O E)C12H21O Ans:B carbo-carbn bondsndicaed od you expettobthonges A)A B)B C)C D)D E)E Ans:D Page 10
Page 10 23. Which structure is different from the others? A) Cl B) CH3CHClCH(CH3)2 C) CH3 C C Cl CH3 H3C H H D) CH3 CH CH CH3 Cl CH3 E) all are identical Ans: E 24. A fairly common algal metabolite is the compound (-)-geosmin, which imparts a musty odor to water even at concentrations in the ppb range. What is the molecular formula of geosmin? (-)-geosmin CH3 HO CH3 A) C11H20O B) C12H22O C) C11H21O D) C12H20O E) C12H21O Ans: B 25. Which of the carbon-carbon bonds indicated would you expect to be the longest in stilbene? A B C D E A) A B) B C) C D) D E) E Ans: D