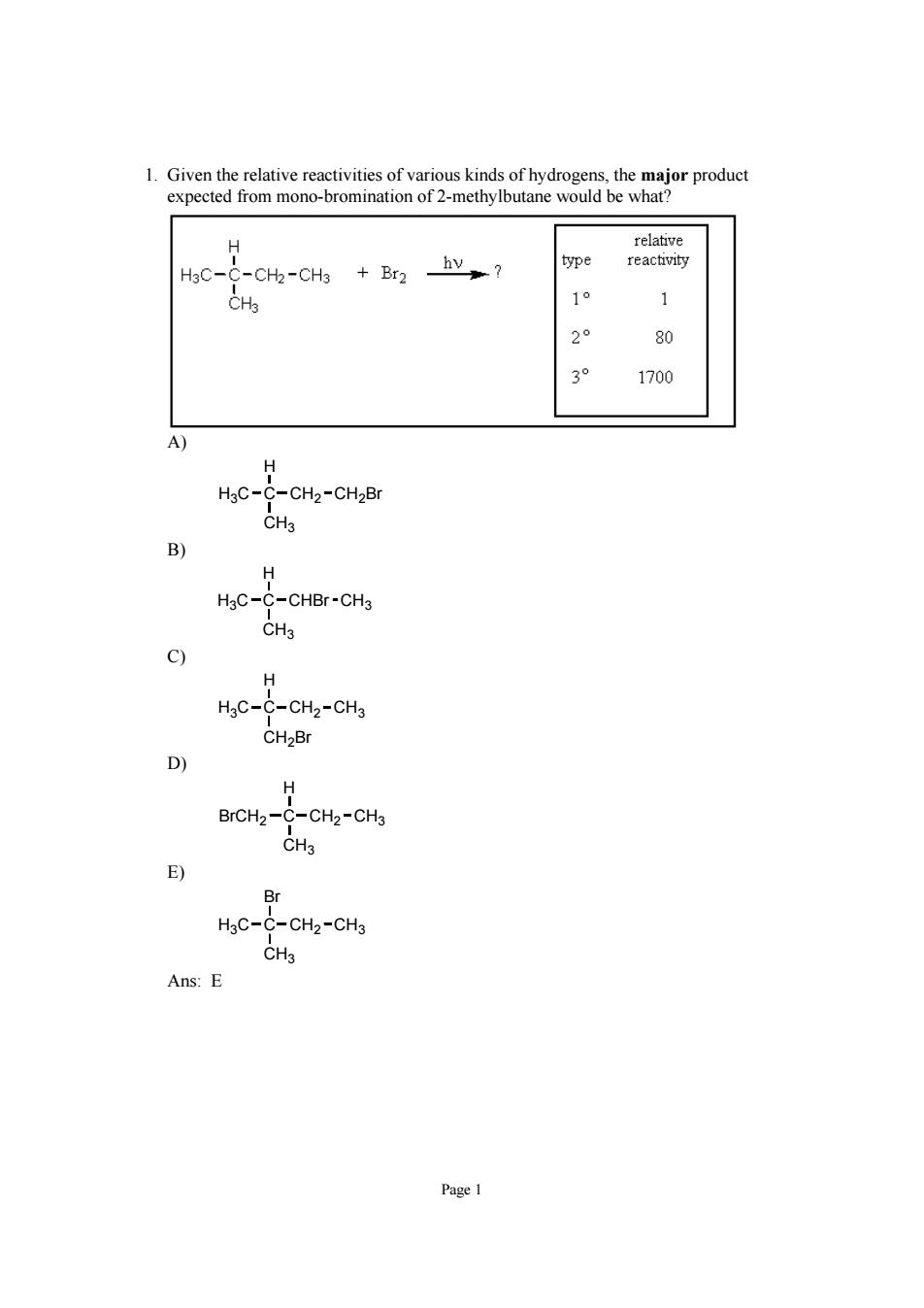
1.Given the relative reactivities of various kinds of hydrogens.the major product expected from mono-bromination of 2-methylbutane would be what H3C- -CH2-CH3 +Br2 hv? 1° 1 80 1700 -CH2-CH2Br CHa B) H HgC-C-CHBr-CH3 CH3 9 H HgC-C-CH2-CH3 CH2Br H BrCH2-C-CH2-CH3 E) Br HaC-C-CHz-CHj CH3 Ans:E
Page 1 1. Given the relative reactivities of various kinds of hydrogens, the major product expected from mono-bromination of 2-methylbutane would be what? A) H3C C CH3 H CH2 CH2Br B) H3C C CH3 H CHBr CH3 C) H3C C CH2Br H CH2 CH3 D) BrCH2 C CH3 H CH2 CH3 E) H3C C CH3 Br CH2 CH3 Ans: E
2.Which of the following is a propagation step in the free-radical bromination of B) Bra hy 2Br. C)CH3+Br2- CH;Br Br. D)CHy+CH: CH3CH3 E)Br?+Br? Br2 Ans:C 3.Which of the reactions below would you expect to have the largest positive A? A) CH:Br B) Br2 hy 2 Br. c) CH3 Br2- CH;Br Br. D) CH3CH3 →CH,CH3 E)Br?+Br? Br2 Ans:B 4.What reactant is needed to complete and balance the following reaction? +302spark 3CO +3H0 A)C3Hs B)C2H6 C)C:Ha D)C:H6 E)CH2 Ans:D 5hnenscsbdwnnomgeyawcomtpgti。 A)CH3 I?CHjl B) 2hv+2. C)CH+I- →CH,I+I D)CH+一 →CH?+HⅢ E)I?+I?- 12 Ans:D Page2
Page 2 2. Which of the following is a propagation step in the free-radical bromination of methane? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: C 3. Which of the reactions below would you expect to have the largest positive Δ? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: B 4. What reactant is needed to complete and balance the following reaction? ? + 3 O2 3 CO + 3 H2O spark A) C3H8 B) C2H6 C) C3H4 D) C3H6 E) C6H12 Ans: D 5. Which one of the reactions below will not proceed and accounts for why iodine added to a free-radical chlorination or bromination will greatly slow or even stop the reaction? A) CH3• + I? CH3I B) hν I2 2 I• C) CH3 CH I + I• 3• + I2 D) CH4 + I• CH3? + HI E) I? + I? I2 Ans: D

6.Which of the following is not true of free-radical halogenation reactions? n chlorine in these reactions. s more s minations are faster emperatures to proceed These reactions are irreversible. Ans:D 7.Which of the following reaction types are typical of alkanes? on more than one of these is correct titution 8.Alkanes are noted for their(choose one) A)high reactivity with acids. D)lack of reactivity B)toxicity. E)water-solubility odor. Page3
Page 3 6. Which of the following is not true of free-radical halogenation reactions? A) Fluorine is more reactive than chlorine in these reactions. B) Bromine is more selective than chlorine. C) The reactions require either light or high temperatures to proceed. D) Brominations are faster than chlorinations. E) These reactions are irreversible. Ans: D 7. Which of the following reaction types are typical of alkanes? A) addition D) reduction B) elimination E) more than one of these is correct C) radical substitution Ans: C 8. Alkanes are noted for their (choose one) A) high reactivity with acids. D) lack of reactivity. B) toxicity. E) water-solubility. C) intense odor. Ans: D
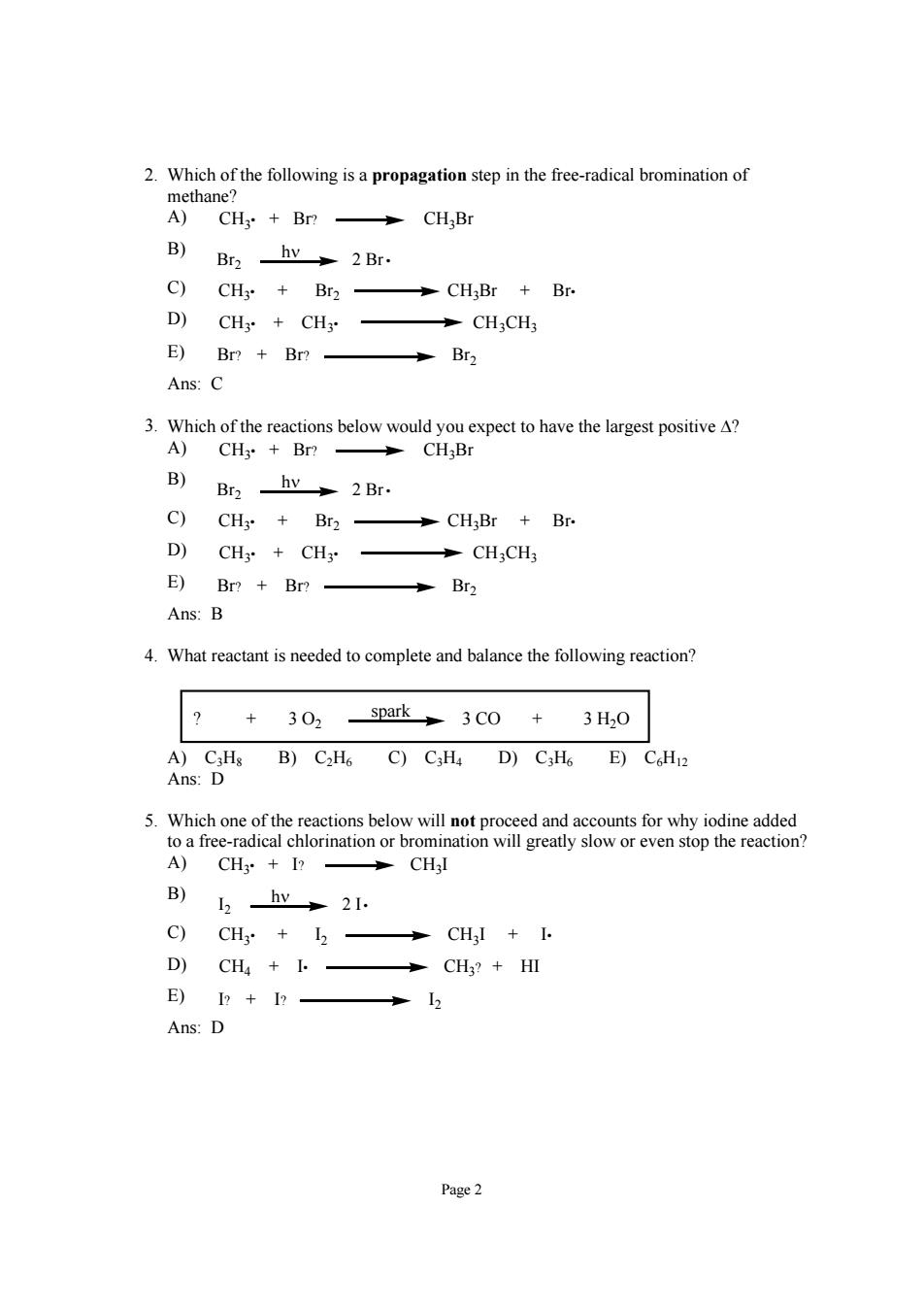
9.What is the major product of the following reaction? C ) Page4
Page 4 9. What is the major product of the following reaction? + Br2 ? light A) Br B) Br C) Br D) Br Br E) no reaction occurs Ans: A
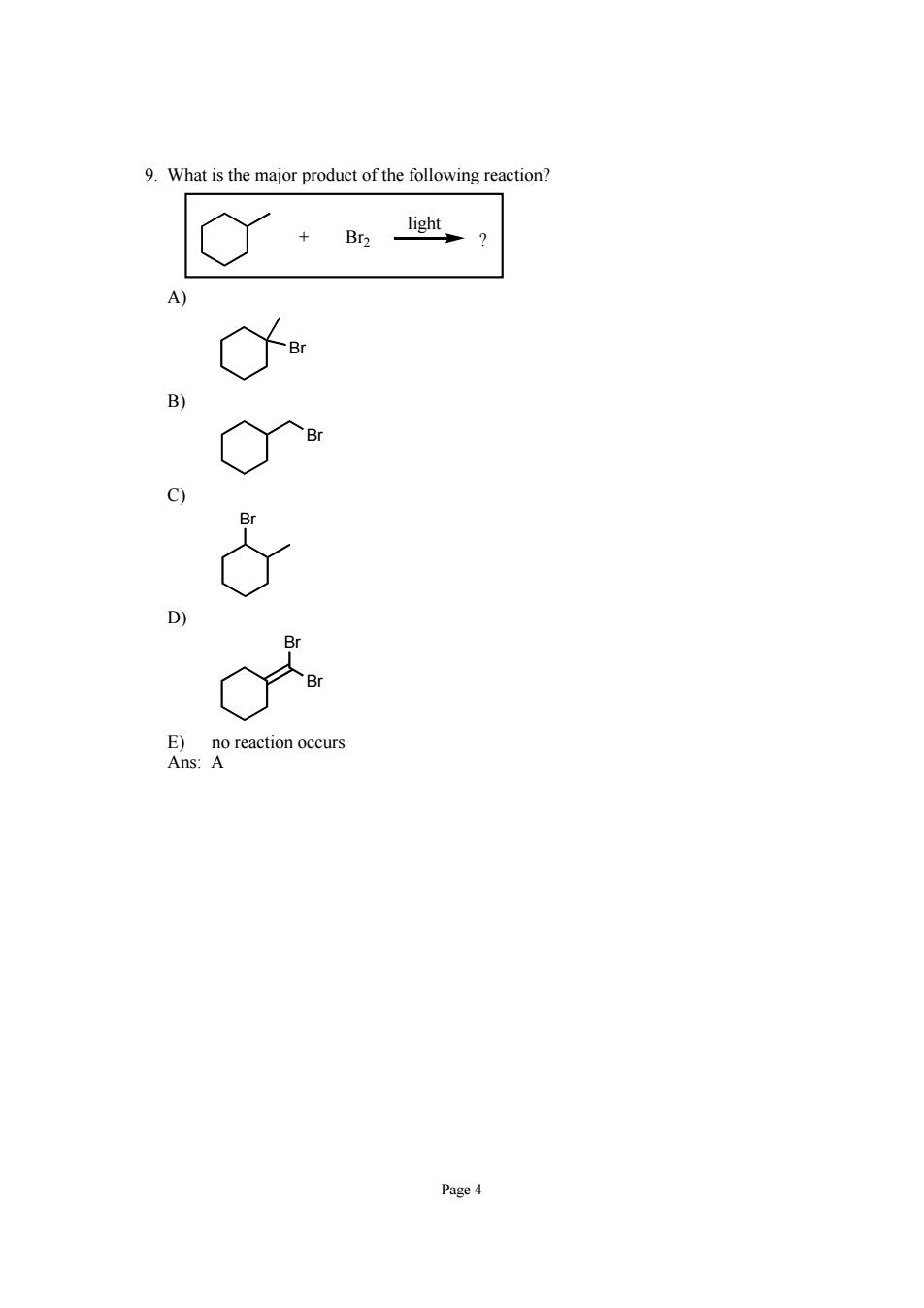
10.Predict the major monofluorination product from the following reaction: C) D) Ans:A 11.In a carbon radical,the single unpaired electron resides in: A)sp'orbital B)sp'orbital C)s orbital D)porbital E)none 12.The order of radical stability is: 3°>20>1>Me 3°<20<1°<Me Mepi all are about equal in stability E)tertiary and methyl are equal in stability Ans:A Page 5
Page 5 10. Predict the major monofluorination product from the following reaction: + ? hυ F2 A) F B) F F C) F D) F E) F Ans: A 11. In a carbon radical, the single unpaired electron resides in: A) sp 2 orbital B) sp 3 orbital C) s orbital D) p orbital E) none of the above Ans: D 12. The order of radical stability is: A) 3° > 2° > 1° > Me B) 3° primary E) tertiary and methyl are equal in stability Ans: A
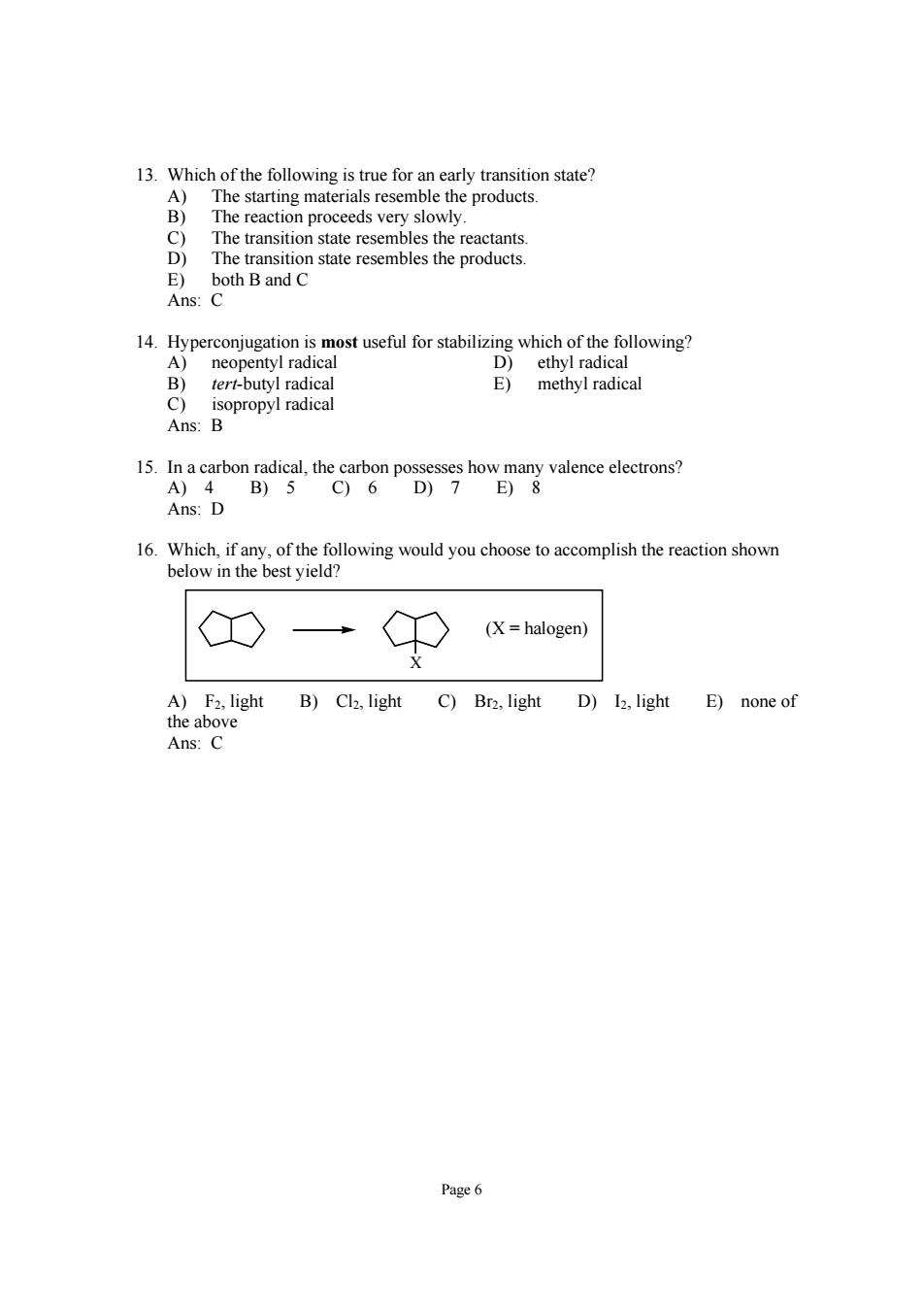
The transition state les the reactants The transition state resembles the products E)both Band C Ans:C ronjation s most useful for stabilizing which of the follow 15.In a carbon radical,the carbon possesses how many valence electrons? A)4 B)5 C)6 D)7 E)8 Ans:D 6ee of the following would you choose to accomplish the reaction showr CO C0 (X=halogen) A)F2.light B)Cl2.light C)Br2.light D)I2.light E)none of the above Ans:C Page6
Page 6 13. Which of the following is true for an early transition state? A) The starting materials resemble the products. B) The reaction proceeds very slowly. C) The transition state resembles the reactants. D) The transition state resembles the products. E) both B and C Ans: C 14. Hyperconjugation is most useful for stabilizing which of the following? A) neopentyl radical D) ethyl radical B) tert-butyl radical E) methyl radical C) isopropyl radical Ans: B 15. In a carbon radical, the carbon possesses how many valence electrons? A) 4 B) 5 C) 6 D) 7 E) 8 Ans: D 16. Which, if any, of the following would you choose to accomplish the reaction shown below in the best yield? X (X = halogen) A) F2, light B) Cl2, light C) Br2, light D) I2, light E) none of the above Ans: C
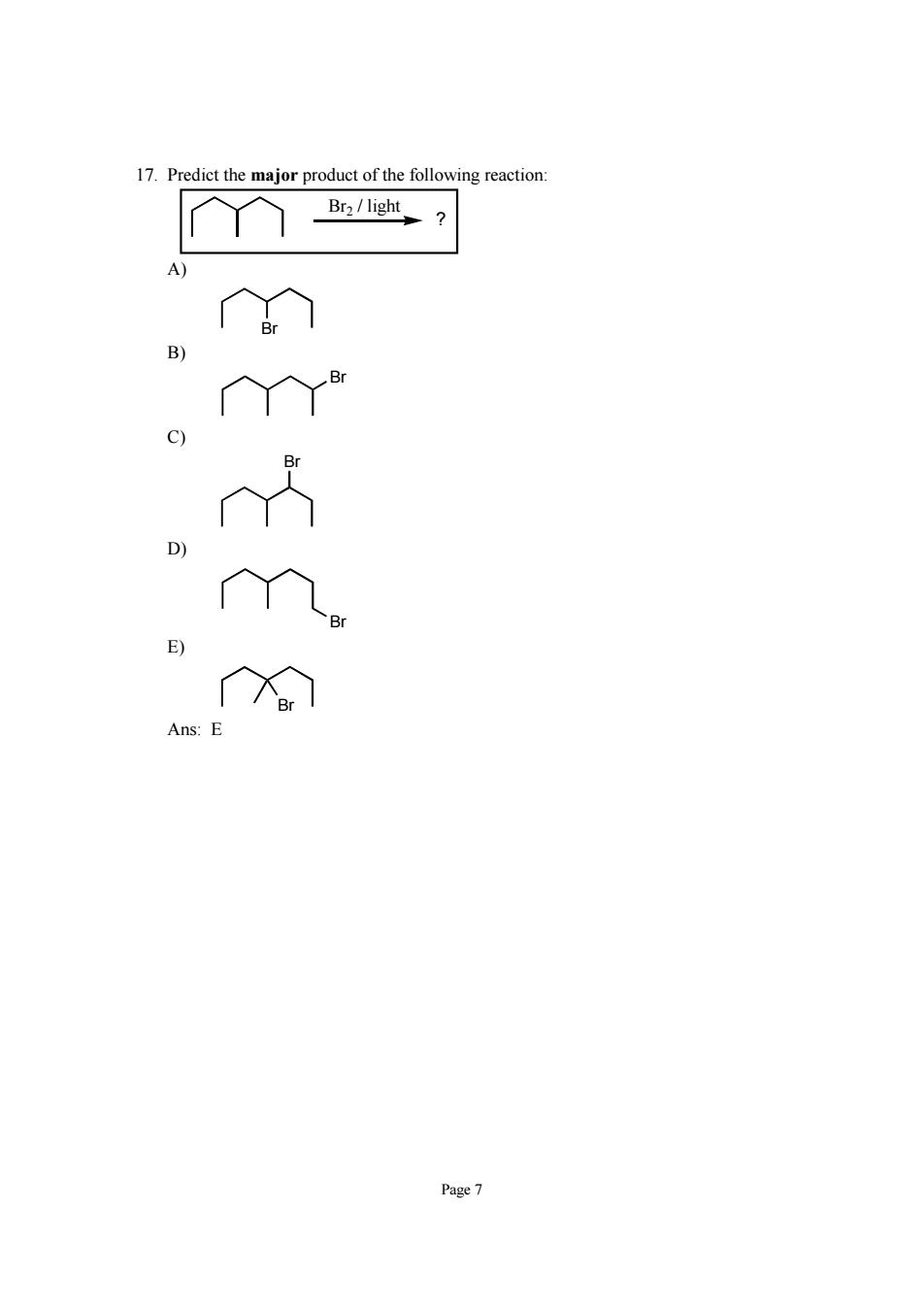
17.Predict the major product of the following reaction: Br2/light C) Page7
Page 7 17. Predict the major product of the following reaction: Br2 / light ? A) Br B) Br C) Br D) Br E) Br Ans: E
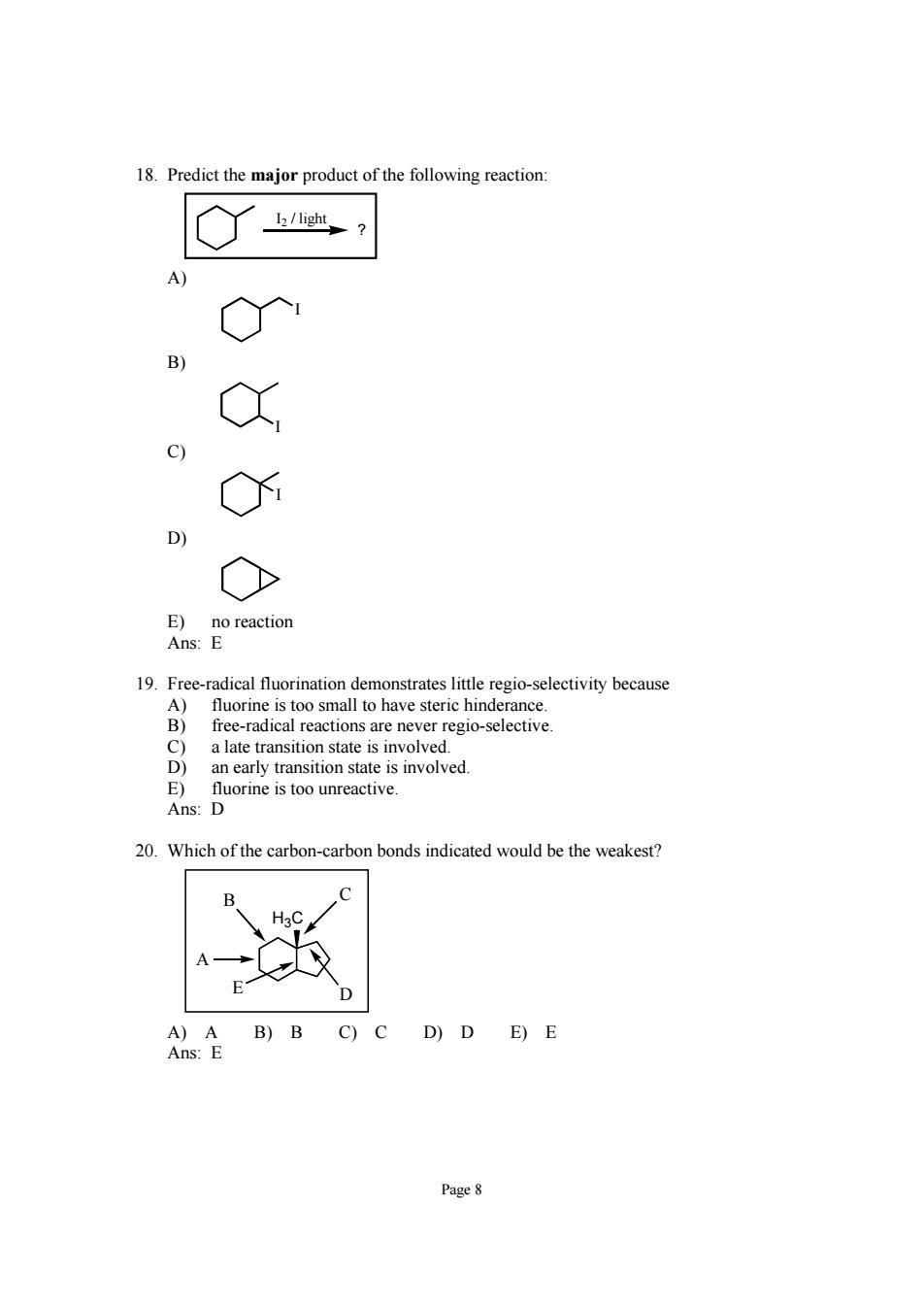
18.Predict the major product of the following reaction 3 E)no reaction Ans:E 19 ueoavctity because o-selective 8 a late transition state is involved. an early transition state is involved. E)fluorine is too unreactive. Ans:D 20.Which of the carbon-carbon bonds indicated would be the weakest? A)A B)B C)C D)D E)E Ans:E Page8
Page 8 18. Predict the major product of the following reaction: I2 / light ? A) I B) I C) I D) E) no reaction Ans: E 19. Free-radical fluorination demonstrates little regio-selectivity because A) fluorine is too small to have steric hinderance. B) free-radical reactions are never regio-selective. C) a late transition state is involved. D) an early transition state is involved. E) fluorine is too unreactive. Ans: D 20. Which of the carbon-carbon bonds indicated would be the weakest? H3C A B C E D A) A B) B C) C D) D E) E Ans: E
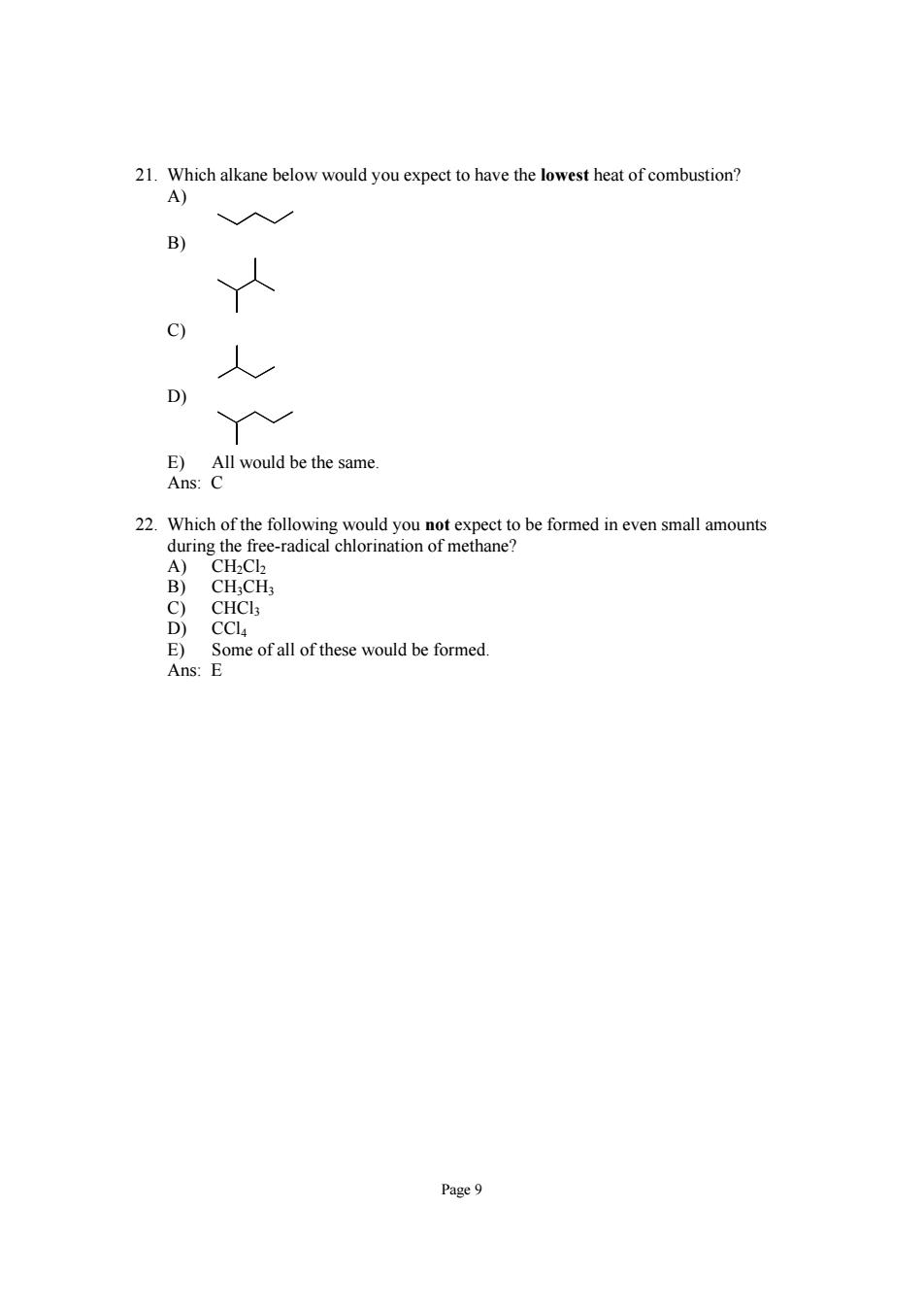
21.Which alkane below would you expect to have the lowest heat of combustion? A) B) C) lwould be the same. 22.Which of the following would you not expect to be formed in even small amounts during the free-radical chlorination of methane? B) CH;CH3 Page9
Page 9 21. Which alkane below would you expect to have the lowest heat of combustion? A) B) C) D) E) All would be the same. Ans: C 22. Which of the following would you not expect to be formed in even small amounts during the free-radical chlorination of methane? A) CH2Cl2 B) CH3CH3 C) CHCl3 D) CCl4 E) Some of all of these would be formed. Ans: E

23.Given the relative reactivities of var ious kinds of hydrogen ected from mono-chlorination of 2-methylbutane would be whpr relative H HgC-C-CH2-CH3 Cl2 hv? type reactivity 3° A) H H3C-C-CH2-CH2Cl CH3 H H3C-C-CHCl-CH3 CH3 9 H HaC-C-CHz-CHa CH2CI D) 9 CHg-C-CH2-CH3 CH3 E)There is no way to predict this. Ans:B Page 10
Page 10 23. Given the relative reactivities of various kinds of hydrogens, the major product expected from mono-chlorination of 2-methylbutane would be what? A) H3C C CH3 H CH2 CH2Cl B) H3C C CH3 H CHCl CH3 C) H3C C CH2Cl H CH2 CH3 D) CH3 C CH3 Cl CH2 CH3 E) There is no way to predict this. Ans: B