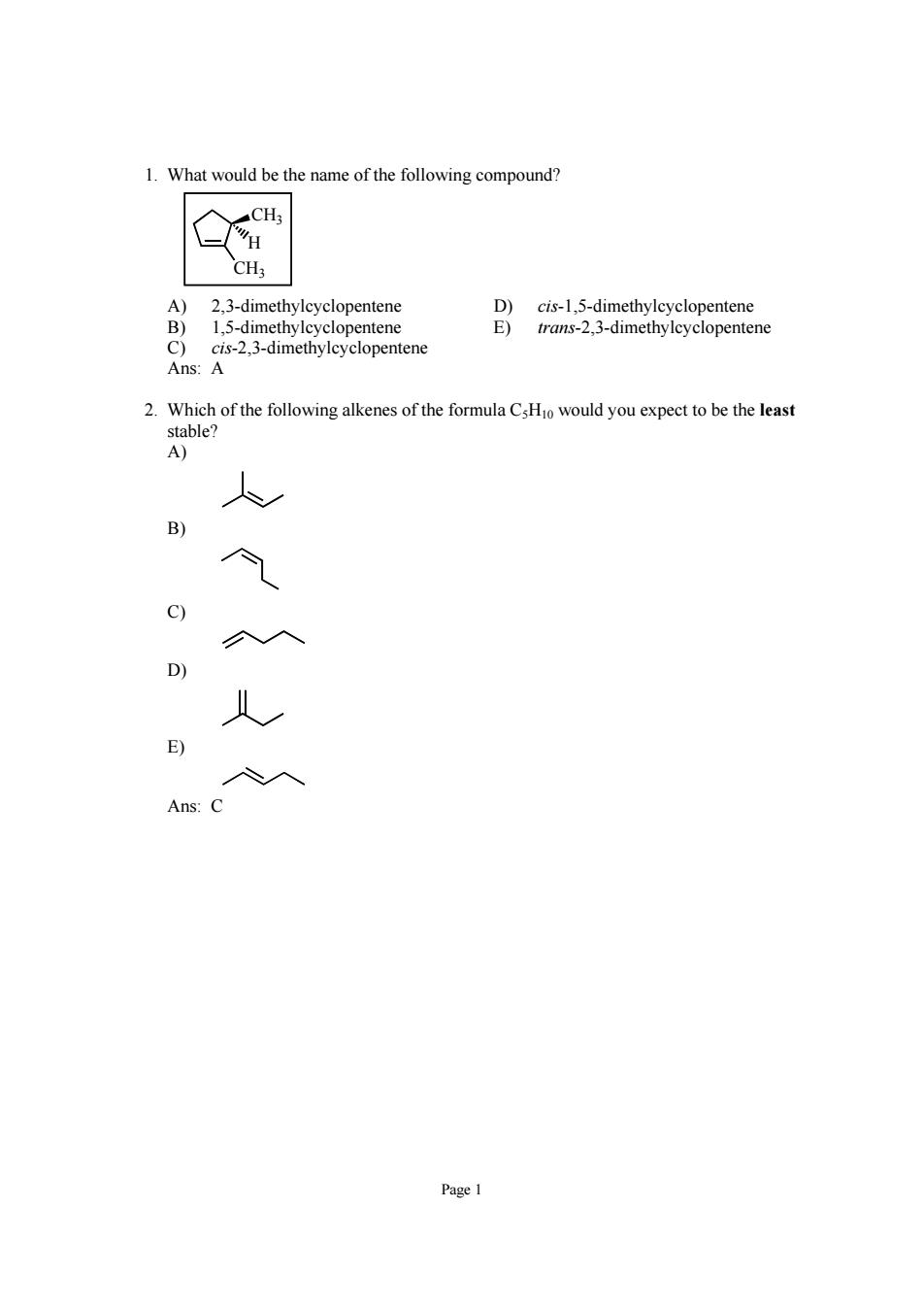
1.What would be the name of the following compound? CHa 2.3-dimethylcyclopentene 15 entene ene Ans:A 2.Which of the following alkenes of the formula CsHo would you expect to be the least stable? A) E Ans:
Page 1 1. What would be the name of the following compound? CH3 CH3 H A) 2,3-dimethylcyclopentene D) cis-1,5-dimethylcyclopentene B) 1,5-dimethylcyclopentene E) trans-2,3-dimethylcyclopentene C) cis-2,3-dimethylcyclopentene Ans: A 2. Which of the following alkenes of the formula C5H10 would you expect to be the least stable? A) B) C) D) E) Ans: C
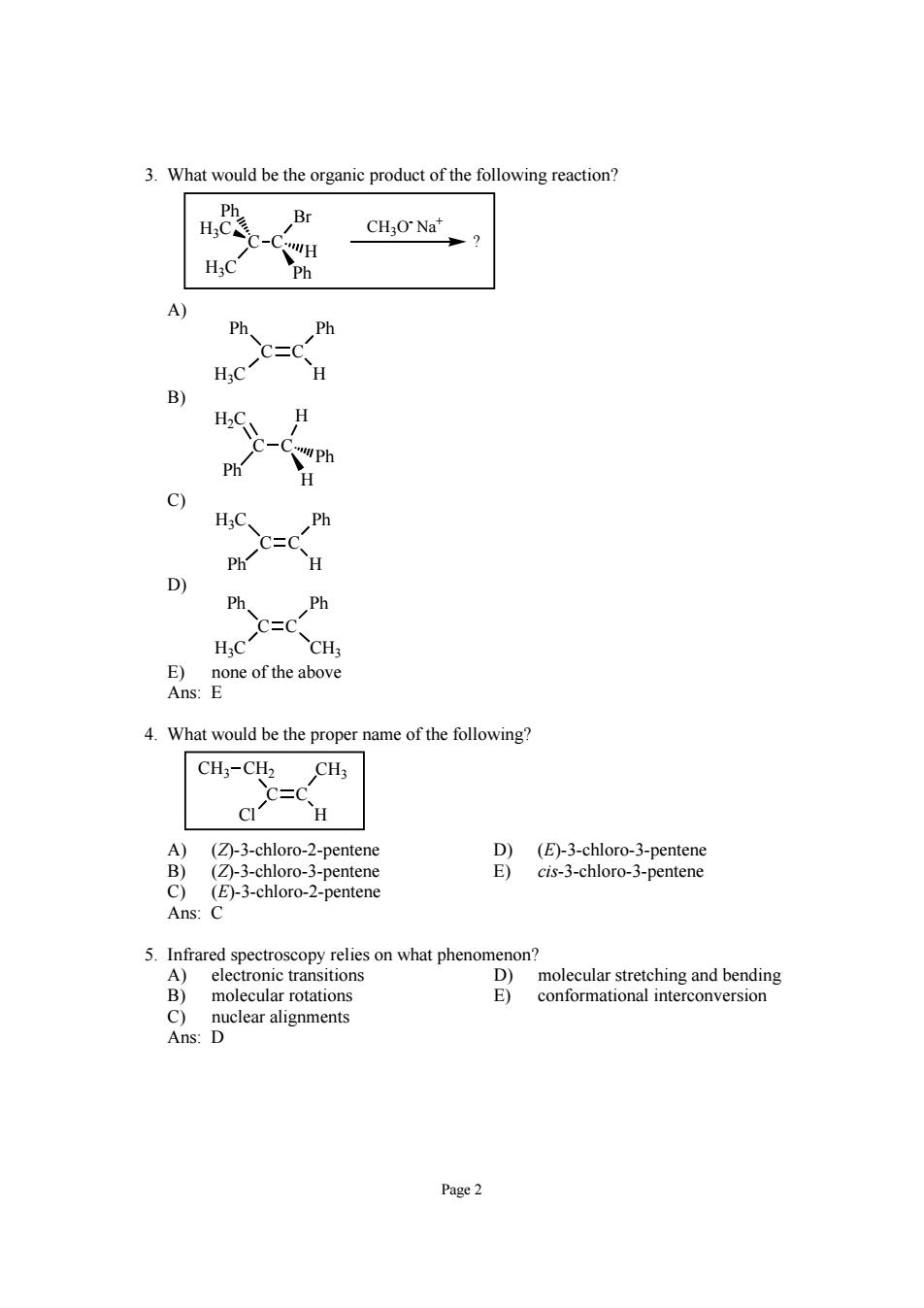
3.What would be the organic product of the following reaction? 4,恐 Br CH3O Na" A C= H HaC\H Ph C) HC、 Ph C=C Ph H Ph Ph C二C H:C CHa one ofthe above 4.What would be the proper name of the following? CH3-CH2 CH3 H A) ②-3 o-2 pentene -3-ch 5.Infrared spectroscopy relies on what phenomenon? D)molecular stretching and bending ar rotatio E) conformational interconversion Page2
Page 2 3. What would be the organic product of the following reaction? C C Ph Br H3C H3C Ph H CH3O- Na+ ? A) C C H Ph Ph H3C B) C C Ph H H 2C H Ph C) C C H H3C Ph Ph D) C C H3C Ph Ph CH3 E) none of the above Ans: E 4. What would be the proper name of the following? C C H CH3 CH2 CH3 Cl A) (Z)-3-chloro-2-pentene D) (E)-3-chloro-3-pentene B) (Z)-3-chloro-3-pentene E) cis-3-chloro-3-pentene C) (E)-3-chloro-2-pentene Ans: C 5. Infrared spectroscopy relies on what phenomenon? A) electronic transitions D) molecular stretching and bending B) molecular rotations E) conformational interconversion C) nuclear alignments Ans: D
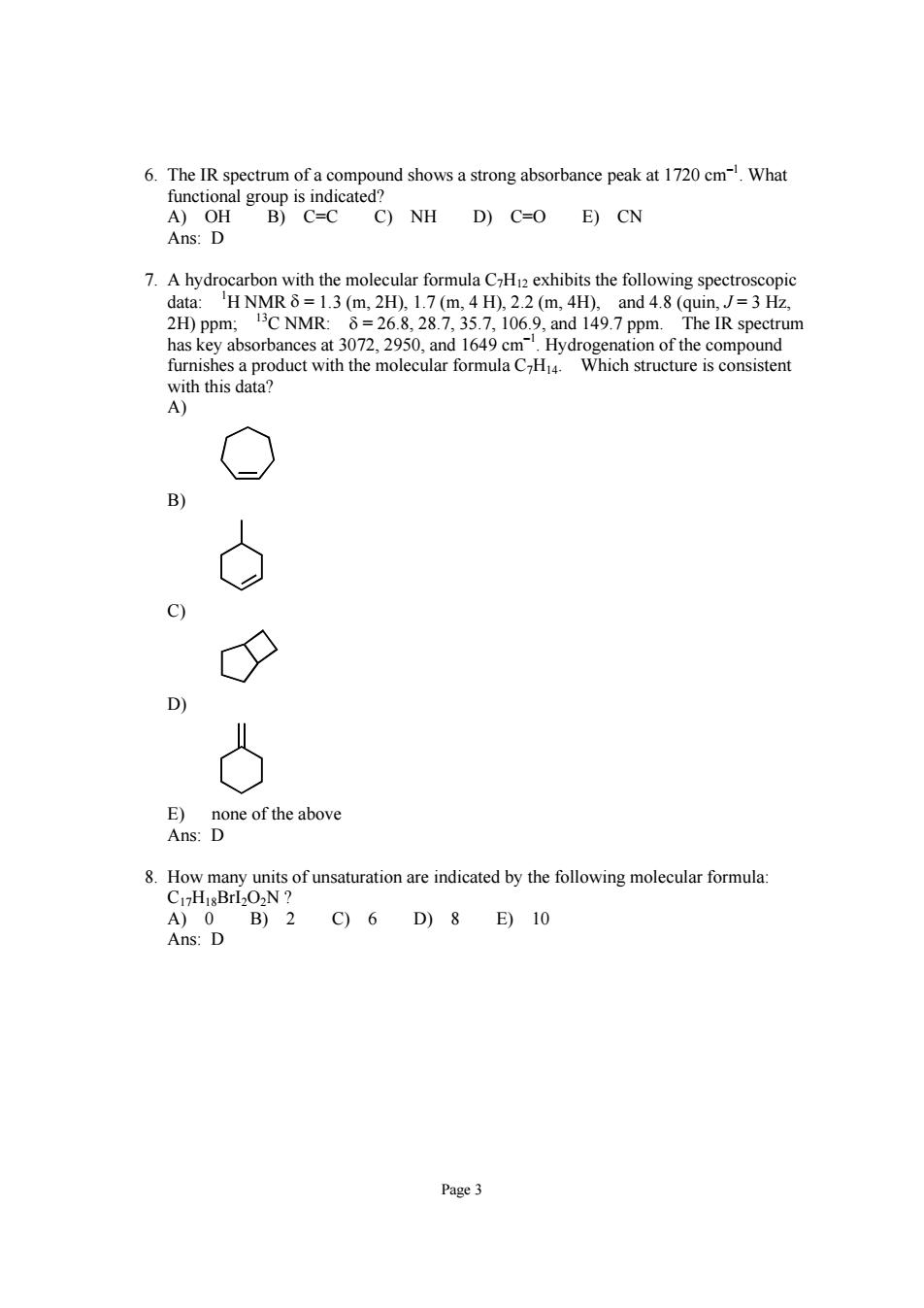
6.The IR spectrum of a compound shows a strong absorbance peak at 1720 cmWhat C)NH D)C=O E)CN 7.A hydrocarbon with the molecular formula CH12 exhibits the following spectroscopic data:H NMR=1.3(m.2H).1.7(m,4 H).2.2(m,4H),and 4.8(quin,J=3Hz. 2H)ppm:C NMR:8=26.8,28.7,35.7,106.9,and 149.7 ppm.The IR spectrum has key absorbances at 3072,2950,and 1649 cm Hydrogenation of the compound furnishes a product with the molecular formula CH4 Which structure is consistent with this data? A) C) D 8.How many units of unsaturation are indicated by the following molecular formula: C1HisBrl2O2N Page3
Page 3 6. The IR spectrum of a compound shows a strong absorbance peak at 1720 cm–1. What functional group is indicated? A) OH B) C=C C) NH D) C=O E) CN Ans: D 7. A hydrocarbon with the molecular formula C7H12 exhibits the following spectroscopic data: 1 H NMR δ = 1.3 (m, 2H), 1.7 (m, 4 H), 2.2 (m, 4H), and 4.8 (quin, J = 3 Hz, 2H) ppm; 13C NMR: δ = 26.8, 28.7, 35.7, 106.9, and 149.7 ppm. The IR spectrum has key absorbances at 3072, 2950, and 1649 cm–1. Hydrogenation of the compound furnishes a product with the molecular formula C7H14. Which structure is consistent with this data? A) B) C) D) E) none of the above Ans: D 8. How many units of unsaturation are indicated by the following molecular formula: C17H18BrI2O2N ? A) 0 B) 2 C) 6 D) 8 E) 10 Ans: D

9.The following molecule contains how many units of unsaturation? HO CO A)2B)3C)5D)6E)7 Ans:E 10.Predict the major product of the following reaction: Hz/Pd-C heat A C) D) H H n pone of the above E) 11.Which of the following most accurately describes the IR fingerprint region: A)2200-3400cm D)1500-1850cm B)600-1500cm E)600-950cm As800-3500 C 12.Which of the following are of lowest energy? A)radio waves B)x-rays C)infirared D)ultraviolet E) microwaves Ans:A Page 4
Page 4 9. The following molecule contains how many units of unsaturation? HO O A) 2 B) 3 C) 5 D) 6 E) 7 Ans: E 10. Predict the major product of the following reaction: H2 / Pd-C ? heat A) O B) C) D) Pd H H E) none of the above Ans: B 11. Which of the following most accurately describes the IR fingerprint region: A) 2200 – 3400 cm–1 D) 1500 – 1850 cm–1 B) 600 – 1500 cm–1 E) 600 – 950 cm–1 C) 3000 – 3500 cm–1 Ans: B 12. Which of the following are of lowest energy? A) radio waves B) x-rays C) infrared D) ultraviolet E) microwaves Ans: A

13.The following molecule contains how many units of unsaturation? FO A)1B)2C)3D)4E)5 Ans:C A)C=O B)C=C C)OH D)NH E)C Ans:E 16.Under specialreaction conditions.-pinene and B-pine e can be i CH, a-pinene B-pinene The a-isomer will predominate. The B-isomer will predominate. The isomers would be equally favored. Ans:A 17.The greatest strength of infrared spectroscopy is in determining A) the degree of unsaturation. the relationships of structural fragments. the functional groups present. the overall structure of the molecule. Ans rings are present. 18.How many degrees of unsaturation are present in the following molecule? C14H13FN20 A)3B)5C)7D)8E)10 Ans:D Page5
Page 5 13. The following molecule contains how many units of unsaturation? O A) 1 B) 2 C) 3 D) 4 E) 5 Ans: C 14. What functional group would be indicated by an IR absorption at 2150 cm–1? A) C=O B) C=C C) OH D) NH E) C C≡ Ans: E 15. What functional group would be indicated by an IR absorption at 1650 cm–1? A) C=O B) C=C C) OH D) NH E) C C≡ Ans: B 16. Under special reaction conditions, α-pinene and β-pinene can be in equilibrium (caused to interconvert reversably). Using your knowledge of alkene stability, which of the following would be true at equilibrium? CH3 CH2 α-pinene β-pinene A) The α-isomer will predominate. B) The β-isomer will predominate. C) The isomers would be equally favored. D) A third isomer would predominate. E) None of the above is true. Ans: A 17. The greatest strength of infrared spectroscopy is in determining A) the degree of unsaturation. B) the relationships of structural fragments. C) the functional groups present. D) the overall structure of the molecule. E) if rings are present. Ans: C 18. How many degrees of unsaturation are present in the following molecule? C14H13F3N2O A) 3 B) 5 C) 7 D) 8 E) 10 Ans: D
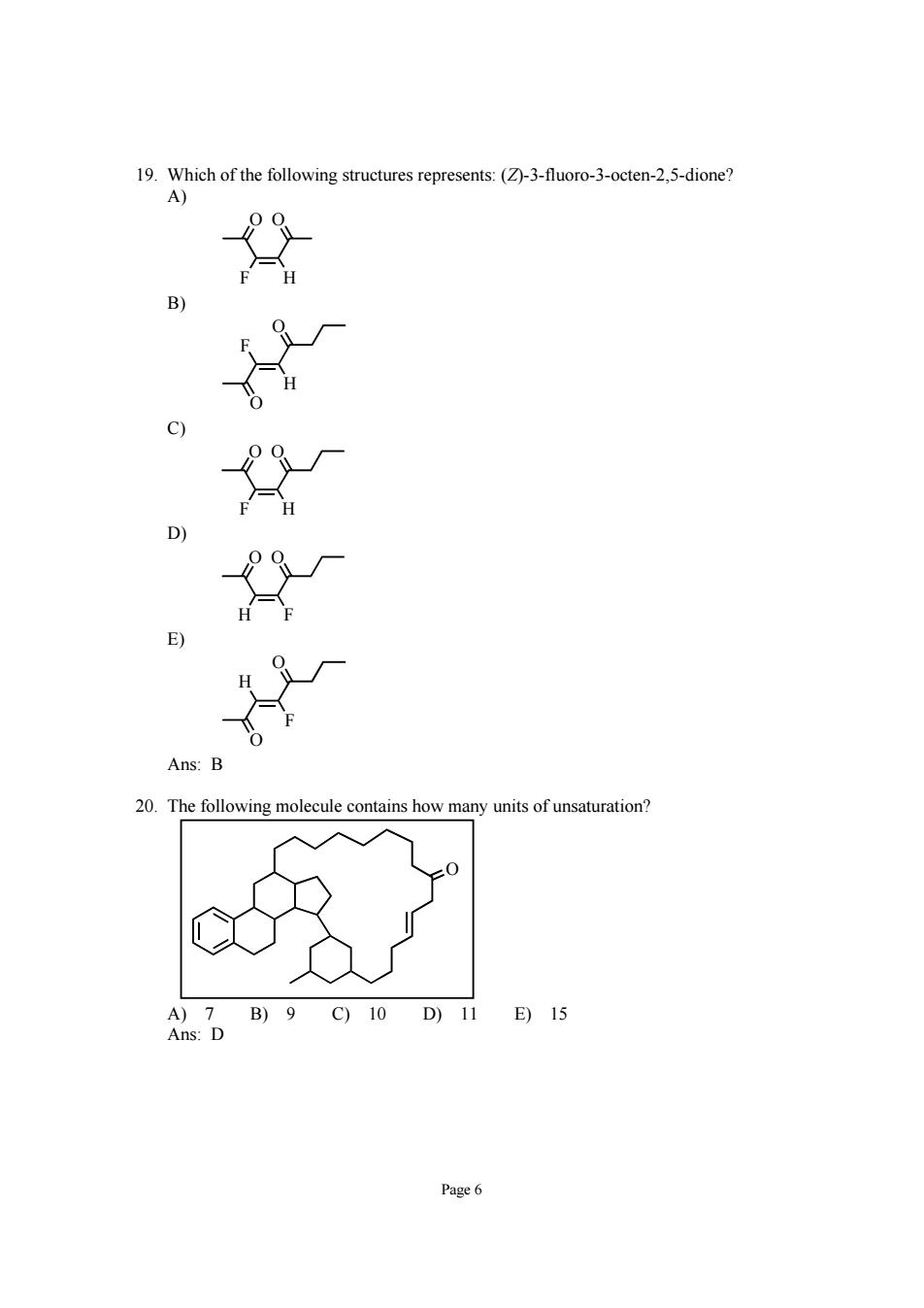
19.Which of the following structures represents:(-3-fuoro-3-octen-2.5-dione? 20.The following molecule contains how many of unsaturation? )7B)9C)10D)11 E)15 Page6
Page 6 19. Which of the following structures represents: (Z)-3-fluoro-3-octen-2,5-dione? A) F H O O B) O H F O C) F H O O D) H F O O E) O F H O Ans: B 20. The following molecule contains how many units of unsaturation? O A) 7 B) 9 C) 10 D) 11 E) 15 Ans: D
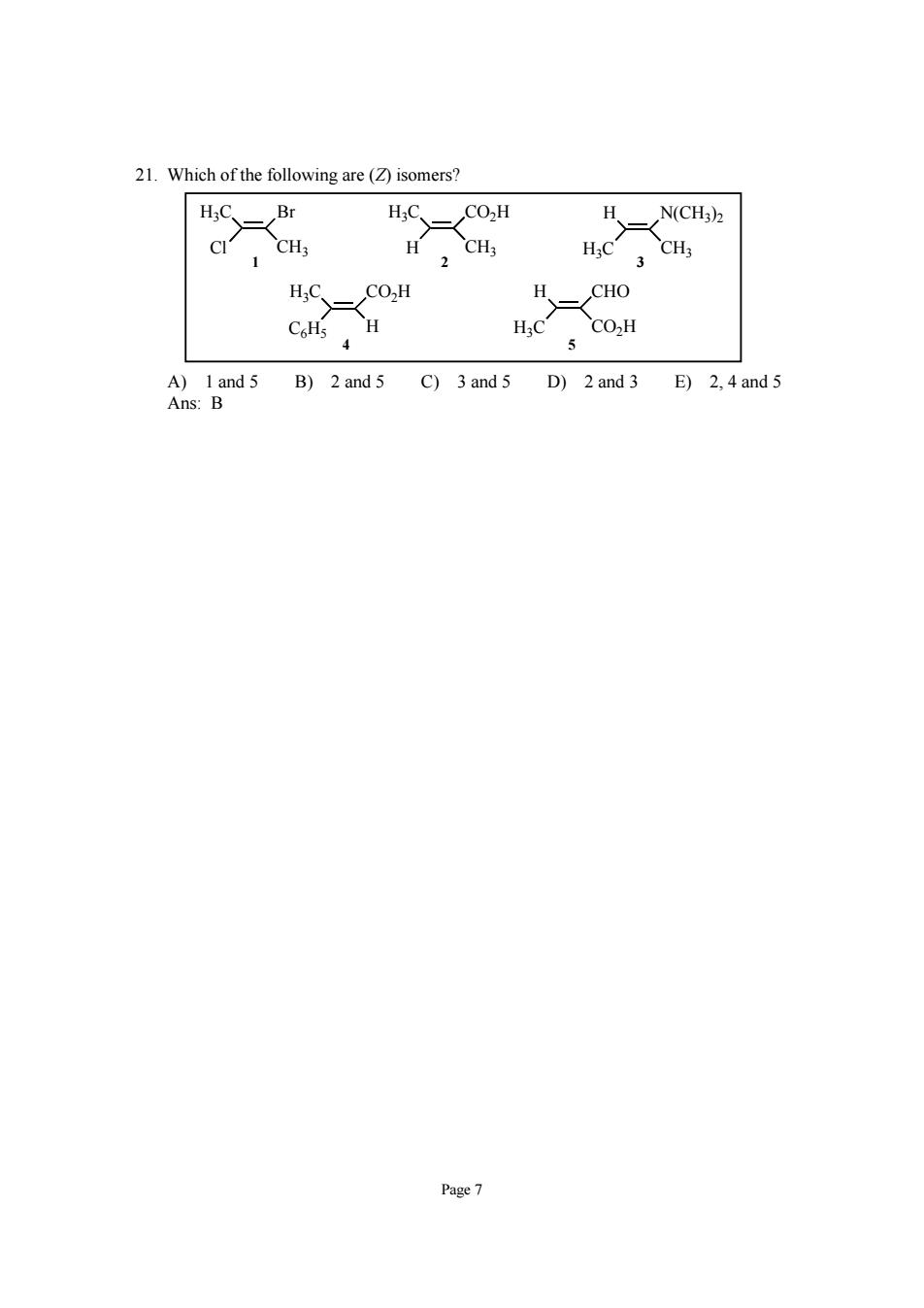
21.Which of the following are(isomers? H;CBr HN(CH3)2 H.C CH H;C CO2H gind 5 B)2and 5 C)3 and 5 D)2 and3 E)2.and5 Page7
Page 7 21. Which of the following are (Z) isomers? H3C Cl Br CH3 H3C H CO2H CH3 H H3C CHO CO2H H3C C6H5 CO2H H H H3C N(CH3)2 CH3 4 5 1 2 3 A) 1 and 5 B) 2 and 5 C) 3 and 5 D) 2 and 3 E) 2, 4 and 5 Ans: B
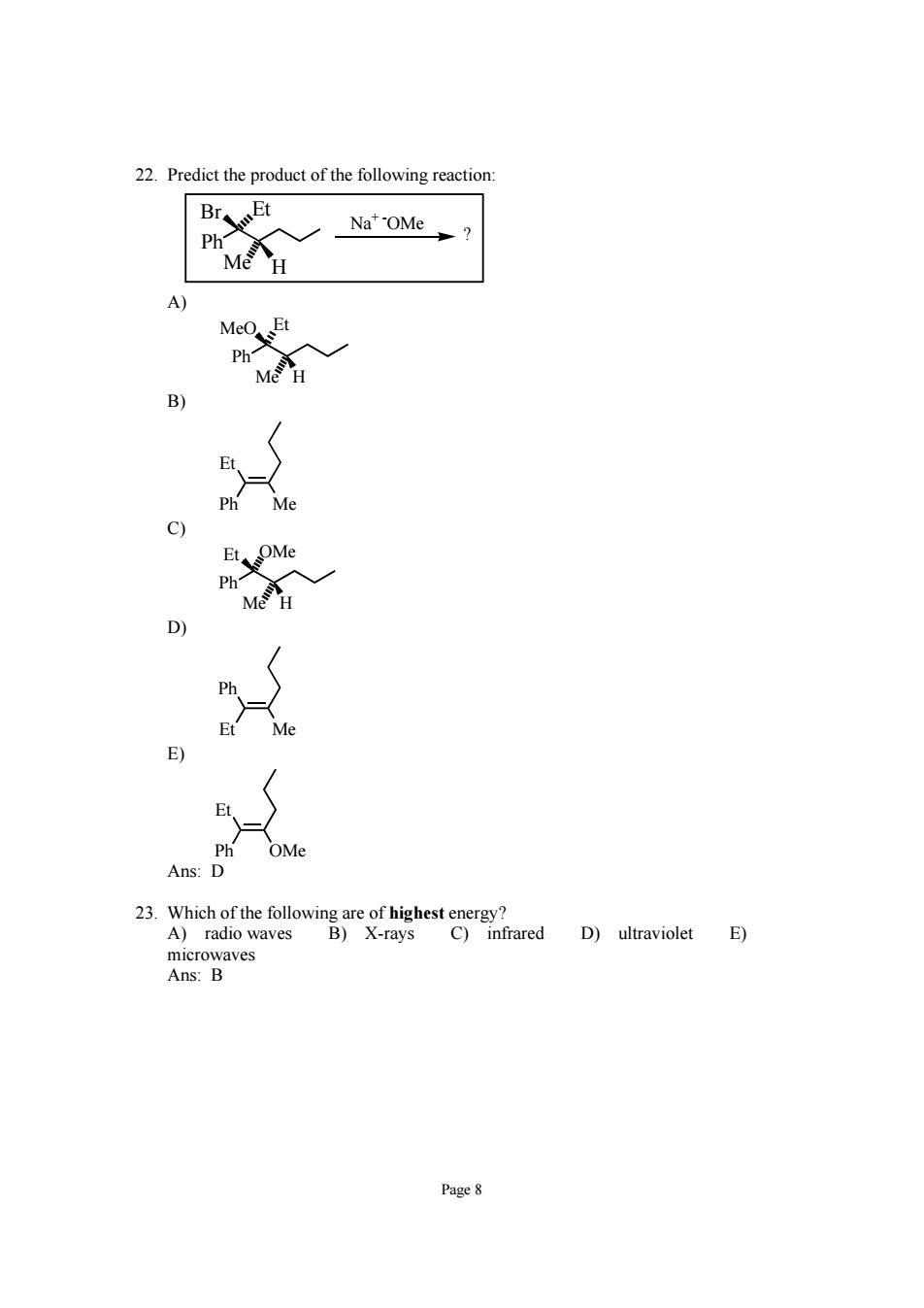
22.Predict the product of the following reaction BrsEt Nat'OMe Ph Me H A MeO Et Ph" M D OM 2及t"dD)raviolet目 A)radio waves microwaves Ans:B Page 8
Page 8 22. Predict the product of the following reaction: Ph Br Et Me H Na+ -OMe ? A) Ph MeO Et Me H B) Ph Me Et C) Ph Et OMe Me H D) Et Me Ph E) Ph OMe Et Ans: D 23. Which of the following are of highest energy? A) radio waves B) X-rays C) infrared D) ultraviolet E) microwaves Ans: B

24.Which is the structure of 3.7-dimethyl-2.6-octadienal? A) CH-OH 3 CH; LCHO CHO Page9
Page 9 24. Which is the structure of 3,7-dimethyl-2,6-octadienal? A) CH2OH CH3 B) CHO CH3 C) CHO CH3 D) CHO CH3 E) CH2OH Ans: D
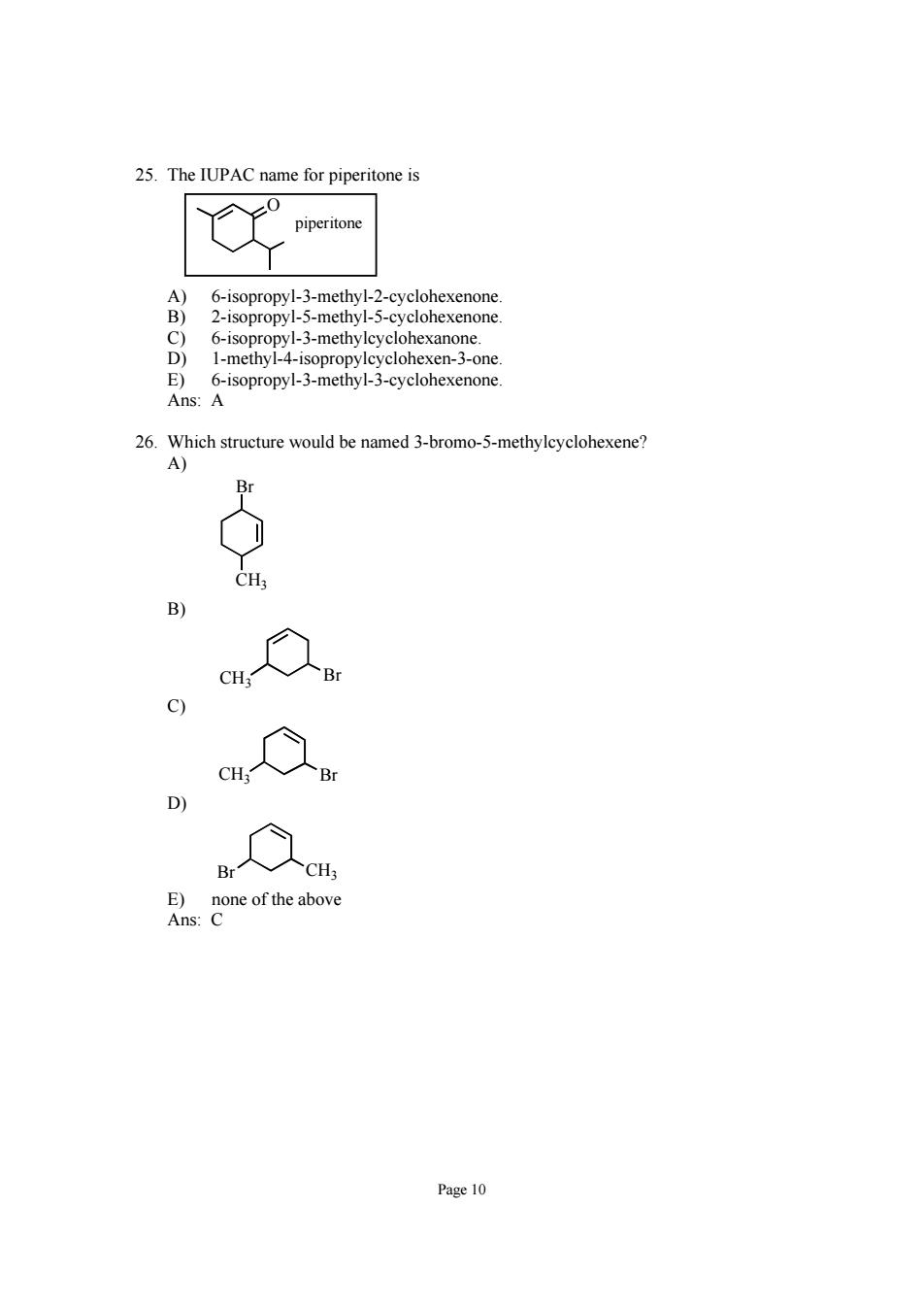
25.The IUPAC name for piperitone is piperitone A) 6-isopropyl-3-methyl-2-cyclohexenone B) 2-isopropyl-5-methyl-5-cyclohexenone c) 6-isopropyl-3-methylcyclohexanone. D) 1-methyl-4-isopropylcyclohexen-3-one. E) 6-isopropyl-3-methyl-3-cyclohexenone. Ans:A 26.Which structure would be named 3-bromo-5-methylcyclohexene? A) Br CH B) CH Br CH D) Br 人CH E) none of the above Ans:C Page 10
Page 10 25. The IUPAC name for piperitone is O piperitone A) 6-isopropyl-3-methyl-2-cyclohexenone. B) 2-isopropyl-5-methyl-5-cyclohexenone. C) 6-isopropyl-3-methylcyclohexanone. D) 1-methyl-4-isopropylcyclohexen-3-one. E) 6-isopropyl-3-methyl-3-cyclohexenone. Ans: A 26. Which structure would be named 3-bromo-5-methylcyclohexene? A) Br CH3 B) CH Br 3 C) CH3 Br D) Br CH3 E) none of the above Ans: C