
Organic Chemistry Exam(B) MidSemester Chinese Name: Student ID: Credit: 1.Name the following compounds in English(IUPAC)(20) (1) 9 2 -CHO 3)入 0 (5) (6)Br ()=CO;H (8)cocI 2.Predict major products for following reactions and indicate the stereochemistry if optional.(20) 1. 1.EtMgBr Ph入 2.NH4CI.H2O 29 HONH2 HGI 3.9 ○+HCHO+EtNH HCI 4.Ph PhP"CH Ph Cr NaOH NH2 Br2 NaOH
1 Organic Chemistry Exam (B) MidSemester Chinese Name: Student ID: Credit: _______________________________________________________________________________ 1. Name the following compounds in English (IUPAC) (20) (1) (2) CHO Cl Br (3) (4) (5) (6) (7) (8) (9) (10) CHO O H N CO2H COCl H CO2Me Cl H O O O Cl OH HO CO2H Br O 2. Predict major products for following reactions and indicate the stereochemistry if optional. (20) 1. EtMgBr 2. NH4Cl, H2O 1. 2. O 3. Ο + HCHO + Et2NH HCl 4. 5. Ph O + Ph3P+CH2Ph Cl- NaOH NH2 H O NaOH Br2 Ph O HONH2 HCl
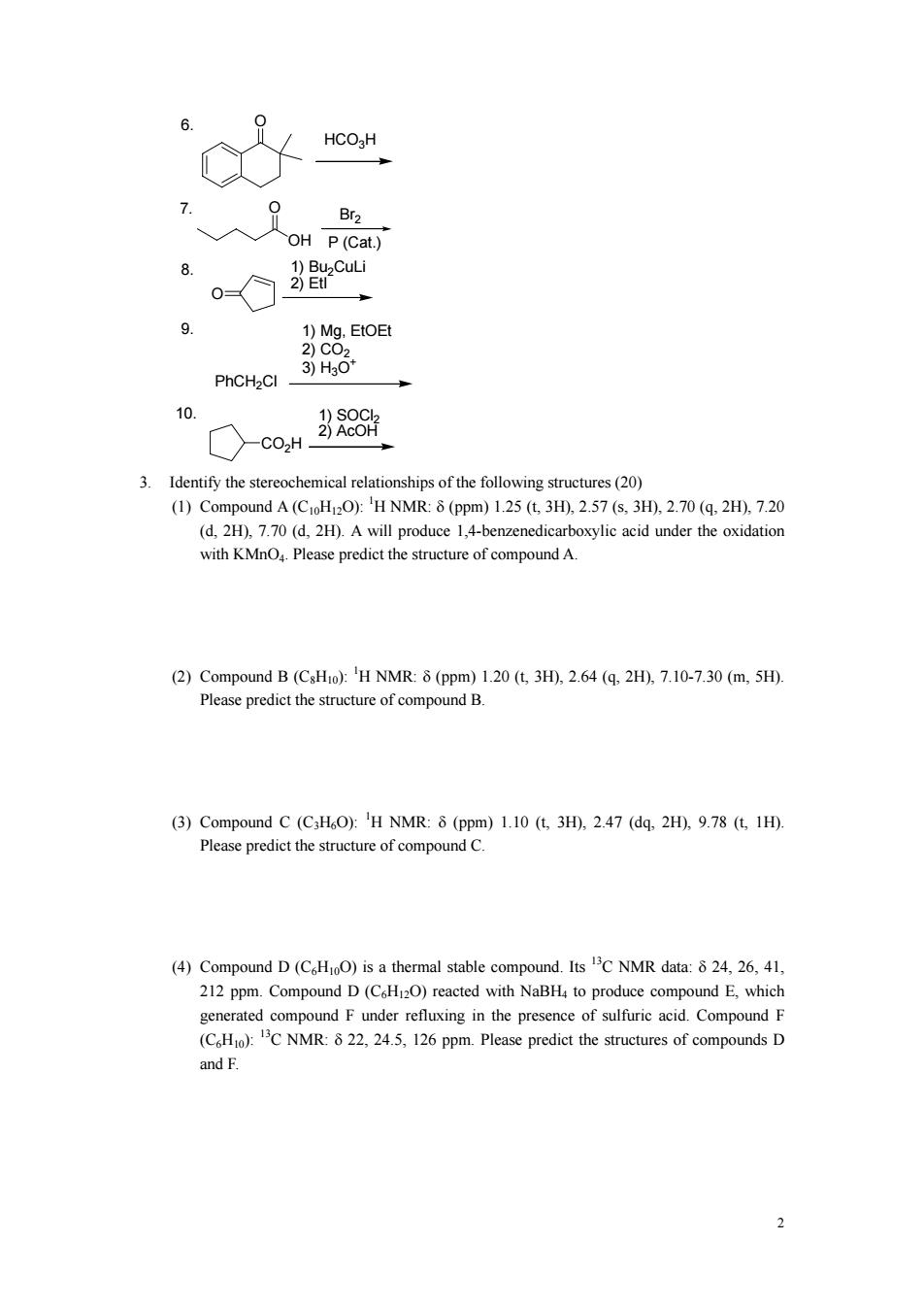
6. 0 HCOgH 9 OH P(Cat) 9. 1)Mg.EtOE 3)H30 10 ○-coH 男80 3.Identify the stereochemical relationships of the following structures(20) (I)Compound A():'H NMR:(ppm)1.25(t3H).2.57(s.3H),2.70(q.2H).7.20 (d,2H),7.70 (d,2H).A will produce 1,4-benzenedicarboxylic acid under the oxidation with KMnO.Please predict the structure of compound A. (2)Compound B(CsHo):'H NMR:8(ppm)10(t3H),2.64 (q.2H),7.10-7.30 (m,5H). Please predict the structure of compound B. (3)Compound C (C3HO):'H NMR:8 (ppm)1.10 (t,3H),2.47 (dq.2H).9.78 (t,1H). Please predict the structure of compound C. (4)Compound D(CHoO)is a thermal stable compound.Its C NMR data:8 24,26,41, 212 ppm.Compound D(CH2O)reacted with NaBH to produce compound E which ted comp ound F under in the presence of sulfuric acid.Compound F (C)CNMR:.24.5.16 ppm.Please predict the structures of compounds D and F
2 6. 7. 8. 9. 10. OH O P (Cat.) Br2 O 1) Bu2CuLi 2) EtI O HCO3H PhCH2Cl 1) Mg, EtOEt 2) CO2 3) H3O+ CO2H 1) SOCl2 2) AcOH 3. Identify the stereochemical relationships of the following structures (20) (1) Compound A (C10H12O): 1 H NMR: δ (ppm) 1.25 (t, 3H), 2.57 (s, 3H), 2.70 (q, 2H), 7.20 (d, 2H), 7.70 (d, 2H). A will produce 1,4-benzenedicarboxylic acid under the oxidation with KMnO4. Please predict the structure of compound A. (2) Compound B (C8H10): 1 H NMR: δ (ppm) 1.20 (t, 3H), 2.64 (q, 2H), 7.10-7.30 (m, 5H). Please predict the structure of compound B. (3) Compound C (C3H6O): 1 H NMR: δ (ppm) 1.10 (t, 3H), 2.47 (dq, 2H), 9.78 (t, 1H). Please predict the structure of compound C. (4) Compound D (C6H10O) is a thermal stable compound. Its 13C NMR data: δ 24, 26, 41, 212 ppm. Compound D (C6H12O) reacted with NaBH4 to produce compound E, which generated compound F under refluxing in the presence of sulfuric acid. Compound F (C6H10): 13C NMR: δ 22, 24.5, 126 ppm. Please predict the structures of compounds D and F

4.Write stepwise and reasonable mechanisms for the following reactions(20). 0入woee= TsOH,PhH 0-0
3 4. Write stepwise and reasonable mechanisms for the following reactions (20). (1) OHC CHO NaOH CHO (2) RCN H3O+ RCO2H O (3) O O MeOH, 100 oC O CO2H O (4) CHO O HOCH2CH2OH O TsOH, PhH
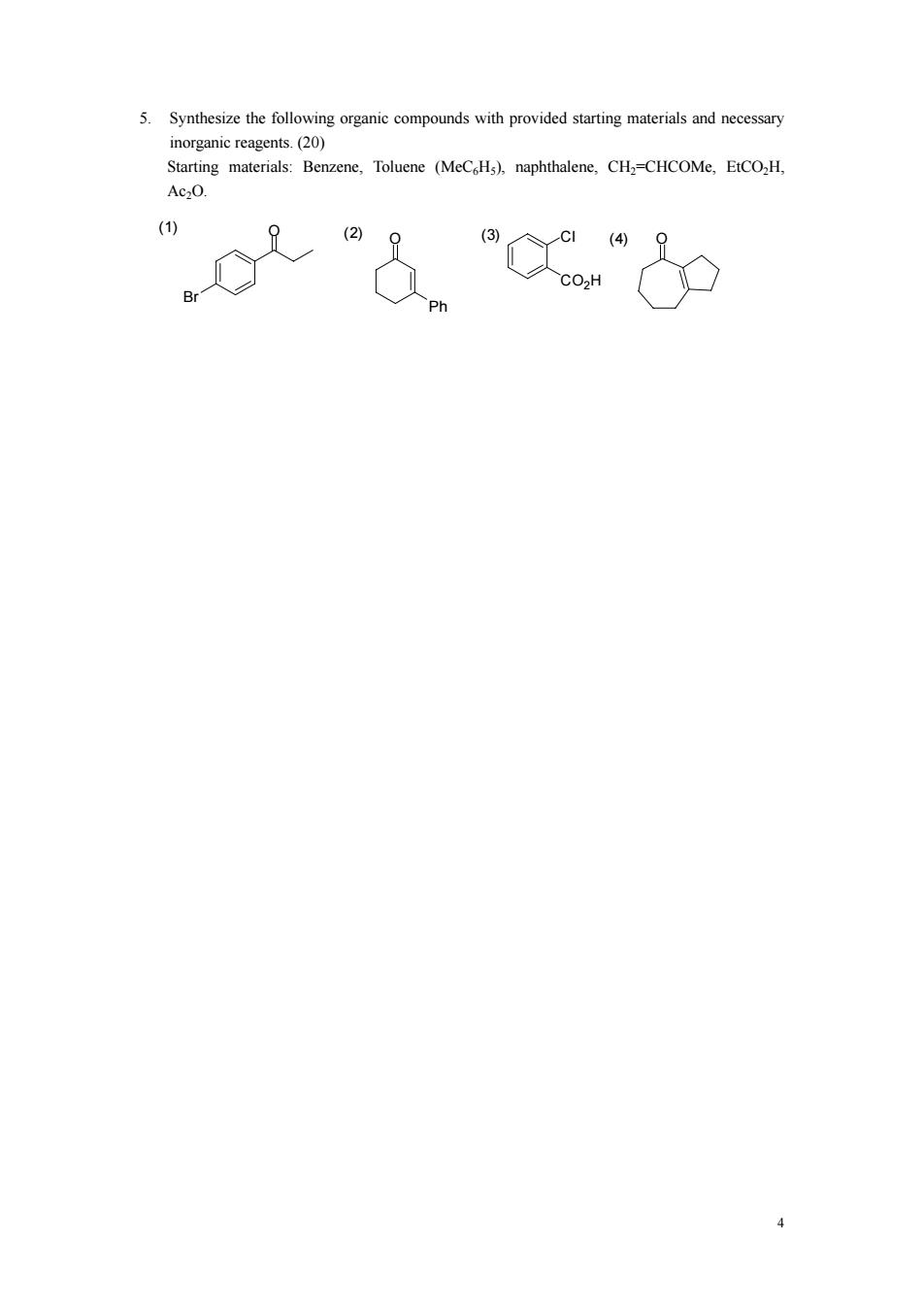
5.Synthesize the following oranic compounds with provided starting materials and necessary inorganic reagents.(20) Starting materials:Benzene,Toluene (MeCH),naphthalene,CH2=CHCOMe,EtCO,H 0".9
4 5. Synthesize the following organic compounds with provided starting materials and necessary inorganic reagents. (20) Starting materials: Benzene, Toluene (MeC6H5), naphthalene, CH2=CHCOMe, EtCO2H, Ac2O. O Ph (1) O Br (2) Cl CO2H (3) (4) O