正在加载图片...
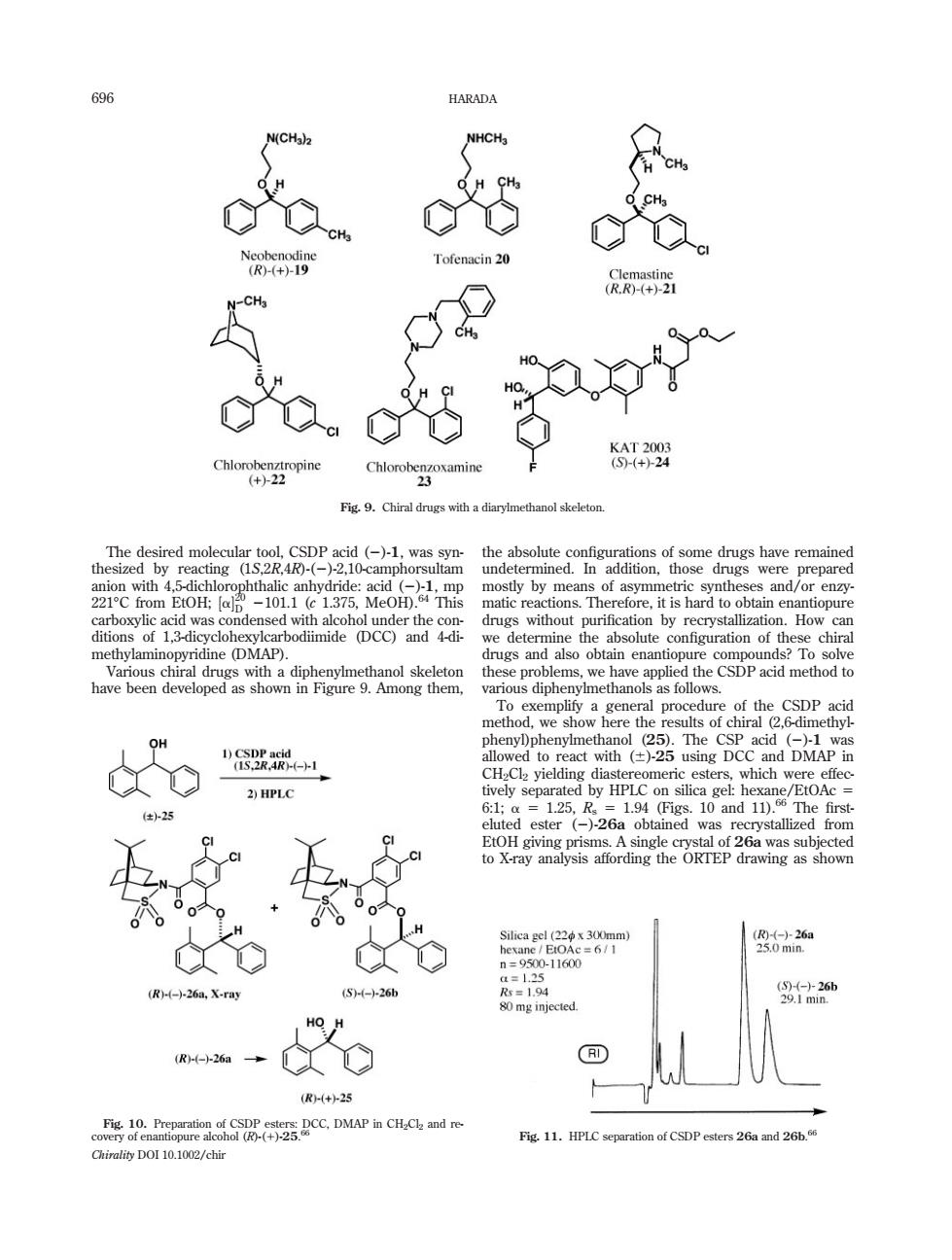
696 HARADA g9 Tofenacin 20 2 Fig.9.Chiral drugs with a D on.How methylamin vridine (DMAP). 1 2)HPLC 25 图62 -11600 (R)(-)-26a,X-ray S--26 80mg injected. HO H 00- D R+25 C.DMAP CHande Fig 11.HPLC separation of CSDP esters 26a and 26b. Chirality DOI 10.1002/chir The desired molecular tool, CSDP acid (2)-1, was synthesized by reacting (1S,2R,4R)-(2)-2,10-camphorsultam anion with 4,5-dichlorophthalic anhydride: acid (2)-1, mp 2218C from EtOH; ½a 20 D 2101.1 (c 1.375, MeOH).64 This carboxylic acid was condensed with alcohol under the conditions of 1,3-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP). Various chiral drugs with a diphenylmethanol skeleton have been developed as shown in Figure 9. Among them, the absolute configurations of some drugs have remained undetermined. In addition, those drugs were prepared mostly by means of asymmetric syntheses and/or enzymatic reactions. Therefore, it is hard to obtain enantiopure drugs without purification by recrystallization. How can we determine the absolute configuration of these chiral drugs and also obtain enantiopure compounds? To solve these problems, we have applied the CSDP acid method to various diphenylmethanols as follows. To exemplify a general procedure of the CSDP acid method, we show here the results of chiral (2,6-dimethylphenyl)phenylmethanol (25). The CSP acid (2)-1 was allowed to react with (6)-25 using DCC and DMAP in CH2Cl2 yielding diastereomeric esters, which were effectively separated by HPLC on silica gel: hexane/EtOAc 5 6:1; a 5 1.25, Rs 5 1.94 (Figs. 10 and 11).66 The firsteluted ester (2)-26a obtained was recrystallized from EtOH giving prisms. A single crystal of 26a was subjected to X-ray analysis affording the ORTEP drawing as shown Fig. 9. Chiral drugs with a diarylmethanol skeleton. Fig. 10. Preparation of CSDP esters: DCC, DMAP in CH2Cl2 and recovery of enantiopure alcohol (R)-(1)-25. 66 Fig. 11. HPLC separation of CSDP esters 26a and 26b. 66 696 HARADA Chirality DOI 10.1002/chir