正在加载图片...
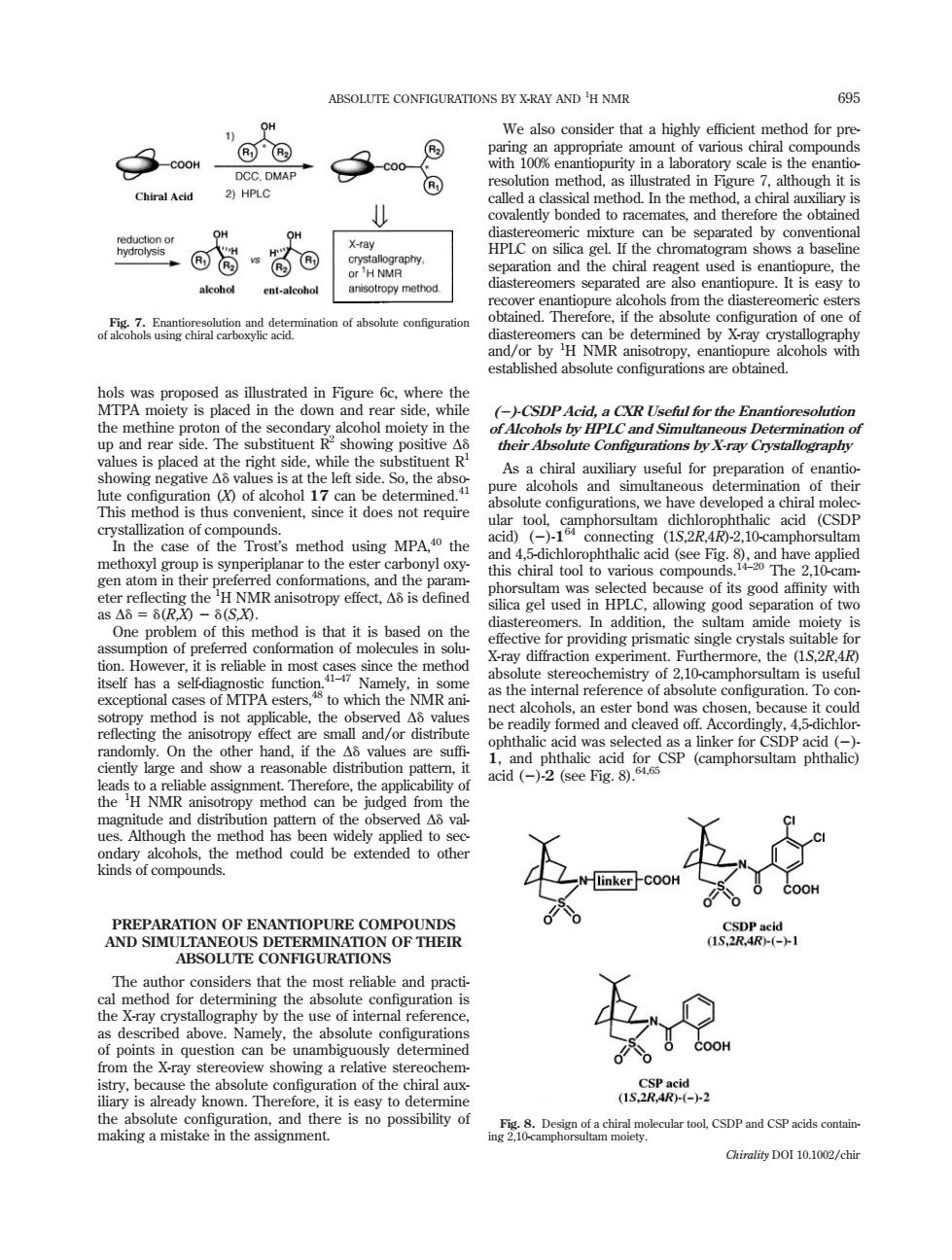
ABSOLUTE CONFIGURATIONS BY X-RAY AND'H NMR 695 OH We also consider that a highly efficient method for pre 回 C DM Chiral Acid 21 HPLC method 店 OH be separated reagent us .the ent-alooho nsotoeymethod hols from the diaste 不hi aination of absolute configuration alcohol moiety in the the p and Simu te Configurations by X-ray Crystallography As a chiral a useful for preparation of ena ve deve oped a chiral gen a sy eed se of its good affinity with a gel us ted bet ddition th ng aparation on th or pre ing pris me 1△8 ntly large a a re able dis ibution pa and phtha the H NMR ani the method could be extended to othe uSR ABSOLUTE CONFIGURATIONS The rthat the most reliableand practi hy by th of points in from the X-ray ais already known.Therefore.it is easy to determine 0s52 n2竖&DaneoabroalcSpmdcpahomtn Chirality DOI 10.1002/chinhols was proposed as illustrated in Figure 6c, where the MTPA moiety is placed in the down and rear side, while the methine proton of the secondary alcohol moiety in the up and rear side. The substituent R2 showing positive Dd values is placed at the right side, while the substituent R1 showing negative Dd values is at the left side. So, the absolute configuration (X) of alcohol 17 can be determined.41 This method is thus convenient, since it does not require crystallization of compounds. In the case of the Trost’s method using MPA,40 the methoxyl group is synperiplanar to the ester carbonyl oxygen atom in their preferred conformations, and the parameter reflecting the 1 H NMR anisotropy effect, Dd is defined as Dd5d(R,X) 2 d(S,X). One problem of this method is that it is based on the assumption of preferred conformation of molecules in solution. However, it is reliable in most cases since the method itself has a self-diagnostic function.41–47 Namely, in some exceptional cases of MTPA esters,48 to which the NMR anisotropy method is not applicable, the observed Dd values reflecting the anisotropy effect are small and/or distribute randomly. On the other hand, if the Dd values are suffi- ciently large and show a reasonable distribution pattern, it leads to a reliable assignment. Therefore, the applicability of the 1 H NMR anisotropy method can be judged from the magnitude and distribution pattern of the observed Dd values. Although the method has been widely applied to secondary alcohols, the method could be extended to other kinds of compounds. PREPARATION OF ENANTIOPURE COMPOUNDS AND SIMULTANEOUS DETERMINATION OF THEIR ABSOLUTE CONFIGURATIONS The author considers that the most reliable and practical method for determining the absolute configuration is the X-ray crystallography by the use of internal reference, as described above. Namely, the absolute configurations of points in question can be unambiguously determined from the X-ray stereoview showing a relative stereochemistry, because the absolute configuration of the chiral auxiliary is already known. Therefore, it is easy to determine the absolute configuration, and there is no possibility of making a mistake in the assignment. We also consider that a highly efficient method for preparing an appropriate amount of various chiral compounds with 100% enantiopurity in a laboratory scale is the enantioresolution method, as illustrated in Figure 7, although it is called a classical method. In the method, a chiral auxiliary is covalently bonded to racemates, and therefore the obtained diastereomeric mixture can be separated by conventional HPLC on silica gel. If the chromatogram shows a baseline separation and the chiral reagent used is enantiopure, the diastereomers separated are also enantiopure. It is easy to recover enantiopure alcohols from the diastereomeric esters obtained. Therefore, if the absolute configuration of one of diastereomers can be determined by X-ray crystallography and/or by 1 H NMR anisotropy, enantiopure alcohols with established absolute configurations are obtained. (2)-CSDP Acid, a CXR Useful for the Enantioresolution of Alcohols by HPLC and Simultaneous Determination of their Absolute Configurations by X-ray Crystallography As a chiral auxiliary useful for preparation of enantiopure alcohols and simultaneous determination of their absolute configurations, we have developed a chiral molecular tool, camphorsultam dichlorophthalic acid (CSDP acid) (2)-164 connecting (1S,2R,4R)-2,10-camphorsultam and 4,5-dichlorophthalic acid (see Fig. 8), and have applied this chiral tool to various compounds.14–20 The 2,10-camphorsultam was selected because of its good affinity with silica gel used in HPLC, allowing good separation of two diastereomers. In addition, the sultam amide moiety is effective for providing prismatic single crystals suitable for X-ray diffraction experiment. Furthermore, the (1S,2R,4R) absolute stereochemistry of 2,10-camphorsultam is useful as the internal reference of absolute configuration. To connect alcohols, an ester bond was chosen, because it could be readily formed and cleaved off. Accordingly, 4,5-dichlorophthalic acid was selected as a linker for CSDP acid (2)- 1, and phthalic acid for CSP (camphorsultam phthalic) acid (2)-2 (see Fig. 8).64,65 Fig. 7. Enantioresolution and determination of absolute configuration of alcohols using chiral carboxylic acid. Fig. 8. Design of a chiral molecular tool, CSDP and CSP acids containing 2,10-camphorsultam moiety. ABSOLUTE CONFIGURATIONS BY X-RAY AND 695 1 H NMR Chirality DOI 10.1002/chir