正在加载图片...
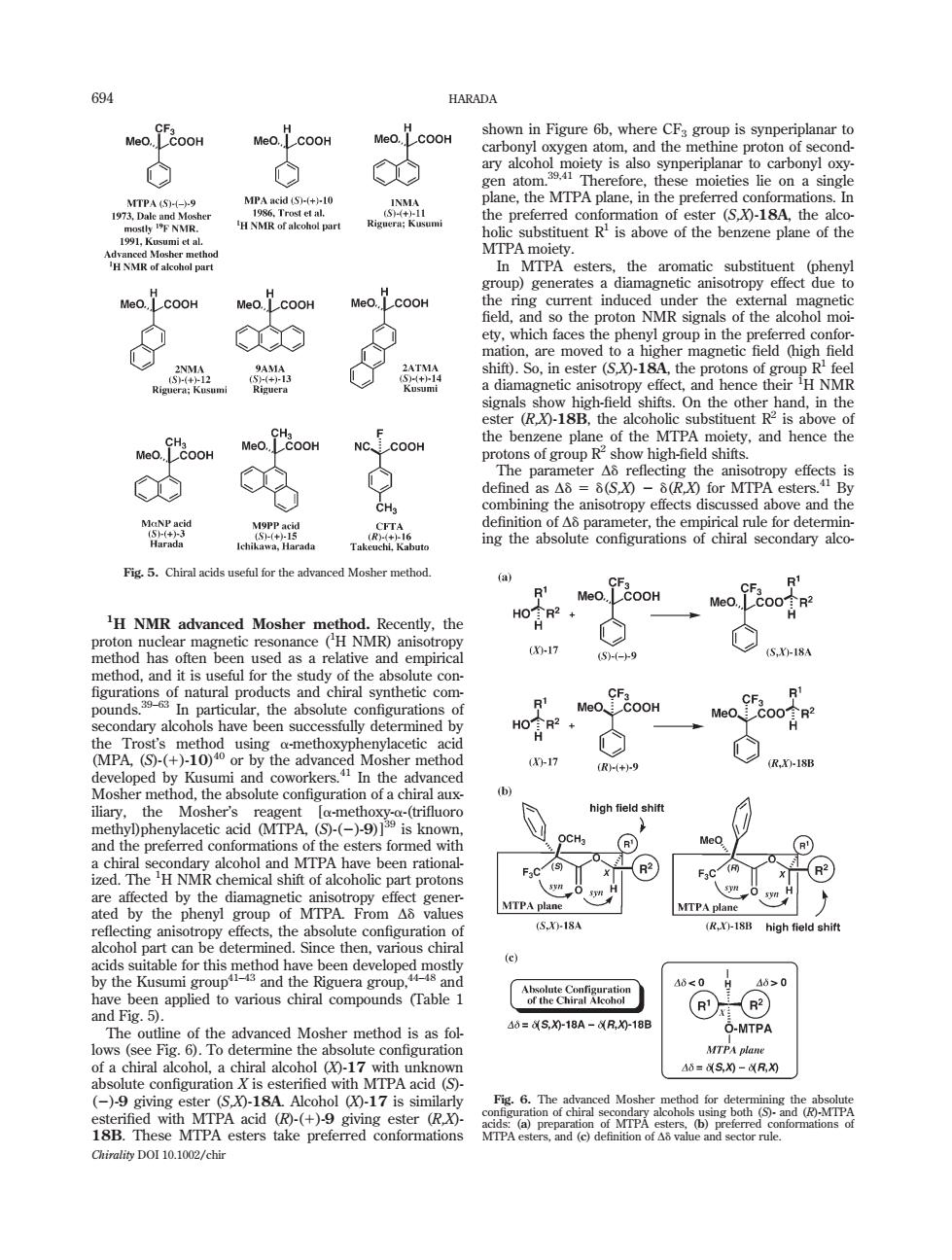
694 HARADA Meo.COOH .COOH H NMR of alcoho Denzene plane Meo.LCOOH Meo.LCOOH aCC shif)are move S-18At出 igh e pro hen c substituentRis aboveo NC COO f eand hence the ◇ the par rou x for Mr py effects i CH. bining g the anisotropy effects discus d above and the Fig5.Chiral acids usefulfor the advanced Mosher method H NMR advanced Mosher m ethod.Recently.the resonance (H NMR anisotropy 5.g 5XH-ISA solute co have been succes fully determined by n-17 luoro red con 品文n院 forme MTPA mis the 18 时he e been develope ed mos ter】 8≤0 d Fig.5) R 40■S,18A-R,018E O-MTPA MTPA pian 40=50-R S0-18A ()-17 is si (R Chirality DOI 10.1002/chir 1 H NMR advanced Mosher method. Recently, the proton nuclear magnetic resonance (1 H NMR) anisotropy method has often been used as a relative and empirical method, and it is useful for the study of the absolute con- figurations of natural products and chiral synthetic compounds.39–63 In particular, the absolute configurations of secondary alcohols have been successfully determined by the Trost’s method using a-methoxyphenylacetic acid (MPA, (S)-(1)-10) 40 or by the advanced Mosher method developed by Kusumi and coworkers.41 In the advanced Mosher method, the absolute configuration of a chiral auxiliary, the Mosher’s reagent [a-methoxy-a-(trifluoro methyl)phenylacetic acid (MTPA, (S)-(2)-9)]39 is known, and the preferred conformations of the esters formed with a chiral secondary alcohol and MTPA have been rationalized. The 1 H NMR chemical shift of alcoholic part protons are affected by the diamagnetic anisotropy effect generated by the phenyl group of MTPA. From Dd values reflecting anisotropy effects, the absolute configuration of alcohol part can be determined. Since then, various chiral acids suitable for this method have been developed mostly by the Kusumi group41–43 and the Riguera group,44–48 and have been applied to various chiral compounds (Table 1 and Fig. 5). The outline of the advanced Mosher method is as follows (see Fig. 6). To determine the absolute configuration of a chiral alcohol, a chiral alcohol (X)-17 with unknown absolute configuration X is esterified with MTPA acid (S)- (2)-9 giving ester (S,X)-18A. Alcohol (X)-17 is similarly esterified with MTPA acid (R)-(1)-9 giving ester (R,X)- 18B. These MTPA esters take preferred conformations shown in Figure 6b, where CF3 group is synperiplanar to carbonyl oxygen atom, and the methine proton of secondary alcohol moiety is also synperiplanar to carbonyl oxygen atom.39,41 Therefore, these moieties lie on a single plane, the MTPA plane, in the preferred conformations. In the preferred conformation of ester (S,X)-18A, the alcoholic substituent R1 is above of the benzene plane of the MTPA moiety. In MTPA esters, the aromatic substituent (phenyl group) generates a diamagnetic anisotropy effect due to the ring current induced under the external magnetic field, and so the proton NMR signals of the alcohol moiety, which faces the phenyl group in the preferred conformation, are moved to a higher magnetic field (high field shift). So, in ester (S,X)-18A, the protons of group R1 feel a diamagnetic anisotropy effect, and hence their 1 H NMR signals show high-field shifts. On the other hand, in the ester (R,X)-18B, the alcoholic substituent R2 is above of the benzene plane of the MTPA moiety, and hence the protons of group R2 show high-field shifts. The parameter Dd reflecting the anisotropy effects is defined as Dd5d(S,X) 2 d(R,X) for MTPA esters.41 By combining the anisotropy effects discussed above and the definition of Dd parameter, the empirical rule for determining the absolute configurations of chiral secondary alcoFig. 5. Chiral acids useful for the advanced Mosher method. Fig. 6. The advanced Mosher method for determining the absolute configuration of chiral secondary alcohols using both (S)- and (R)-MTPA acids: (a) preparation of MTPA esters, (b) preferred conformations of MTPA esters, and (c) definition of Dd value and sector rule. 694 HARADA Chirality DOI 10.1002/chir