正在加载图片...
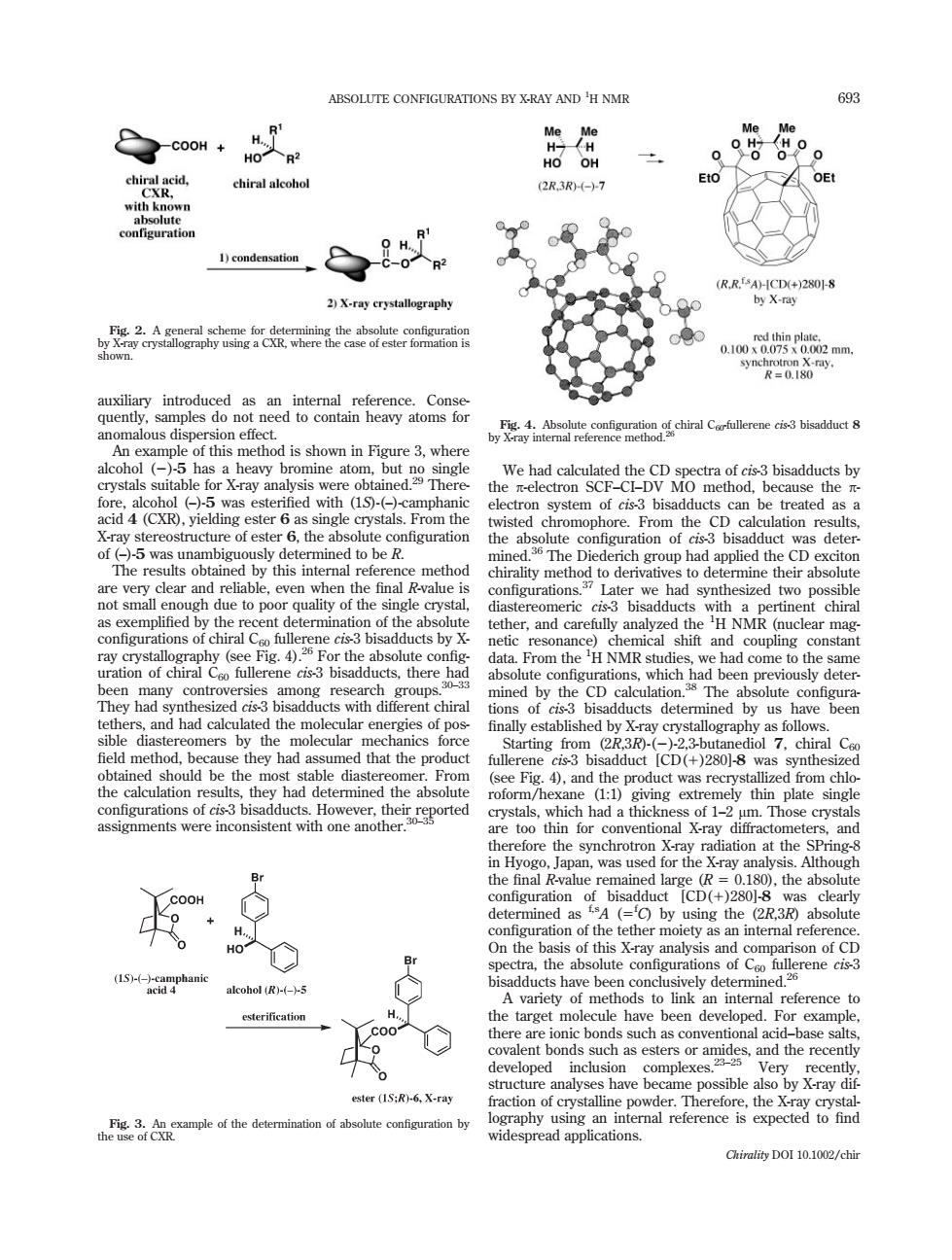
ABSOLUTE CONFIGURATIONS BY X-RAY AND'H NMR 693 HoR2 chiral alcoho 2R.3R7 o 1)condensation 2)X-raycrystallography RRX 2二放 cied with (1- can be treated asa isted the we had sy iastereomeric bisadducts chira otesneG finally d that the product mea品 rotHoeitetgored ntionats of 1-2 m.Those crysta therefore the synchroting was us the f 11 the Althoug COOH of bisa 2R.3R ration of the tether moiety as an inteml refere arget mo ule have been developed. For example and the recent developed complexes ster (IS:R)-6,X-ray raction of crystalline Chirality DOI 10.1002/chirauxiliary introduced as an internal reference. Consequently, samples do not need to contain heavy atoms for anomalous dispersion effect. An example of this method is shown in Figure 3, where alcohol (2)-5 has a heavy bromine atom, but no single crystals suitable for X-ray analysis were obtained.29 Therefore, alcohol (–)-5 was esterified with (1S)-(–)-camphanic acid 4 (CXR), yielding ester 6 as single crystals. From the X-ray stereostructure of ester 6, the absolute configuration of (–)-5 was unambiguously determined to be R. The results obtained by this internal reference method are very clear and reliable, even when the final R-value is not small enough due to poor quality of the single crystal, as exemplified by the recent determination of the absolute configurations of chiral C60 fullerene cis-3 bisadducts by Xray crystallography (see Fig. 4).26 For the absolute configuration of chiral C60 fullerene cis-3 bisadducts, there had been many controversies among research groups.30–33 They had synthesized cis-3 bisadducts with different chiral tethers, and had calculated the molecular energies of possible diastereomers by the molecular mechanics force field method, because they had assumed that the product obtained should be the most stable diastereomer. From the calculation results, they had determined the absolute configurations of cis-3 bisadducts. However, their reported assignments were inconsistent with one another.30–35 We had calculated the CD spectra of cis-3 bisadducts by the p-electron SCF–CI–DV MO method, because the pelectron system of cis-3 bisadducts can be treated as a twisted chromophore. From the CD calculation results, the absolute configuration of cis-3 bisadduct was determined.36 The Diederich group had applied the CD exciton chirality method to derivatives to determine their absolute configurations.37 Later we had synthesized two possible diastereomeric cis-3 bisadducts with a pertinent chiral tether, and carefully analyzed the 1 H NMR (nuclear magnetic resonance) chemical shift and coupling constant data. From the 1 H NMR studies, we had come to the same absolute configurations, which had been previously determined by the CD calculation.38 The absolute configurations of cis-3 bisadducts determined by us have been finally established by X-ray crystallography as follows. Starting from (2R,3R)-(2)-2,3-butanediol 7, chiral C60 fullerene cis-3 bisadduct [CD(1)280]-8 was synthesized (see Fig. 4), and the product was recrystallized from chloroform/hexane (1:1) giving extremely thin plate single crystals, which had a thickness of 1–2 lm. Those crystals are too thin for conventional X-ray diffractometers, and therefore the synchrotron X-ray radiation at the SPring-8 in Hyogo, Japan, was used for the X-ray analysis. Although the final R-value remained large (R 5 0.180), the absolute configuration of bisadduct [CD(1)280]-8 was clearly determined as f,sA (5f C) by using the (2R,3R) absolute configuration of the tether moiety as an internal reference. On the basis of this X-ray analysis and comparison of CD spectra, the absolute configurations of C60 fullerene cis-3 bisadducts have been conclusively determined.26 A variety of methods to link an internal reference to the target molecule have been developed. For example, there are ionic bonds such as conventional acid–base salts, covalent bonds such as esters or amides, and the recently developed inclusion complexes.23–25 Very recently, structure analyses have became possible also by X-ray diffraction of crystalline powder. Therefore, the X-ray crystallography using an internal reference is expected to find widespread applications. Fig. 3. An example of the determination of absolute configuration by the use of CXR. Fig. 4. Absolute configuration of chiral C60-fullerene cis-3 bisadduct 8 by X-ray internal reference method.26 Fig. 2. A general scheme for determining the absolute configuration by X-ray crystallography using a CXR, where the case of ester formation is shown. ABSOLUTE CONFIGURATIONS BY X-RAY AND 693 1 H NMR Chirality DOI 10.1002/chir