正在加载图片...
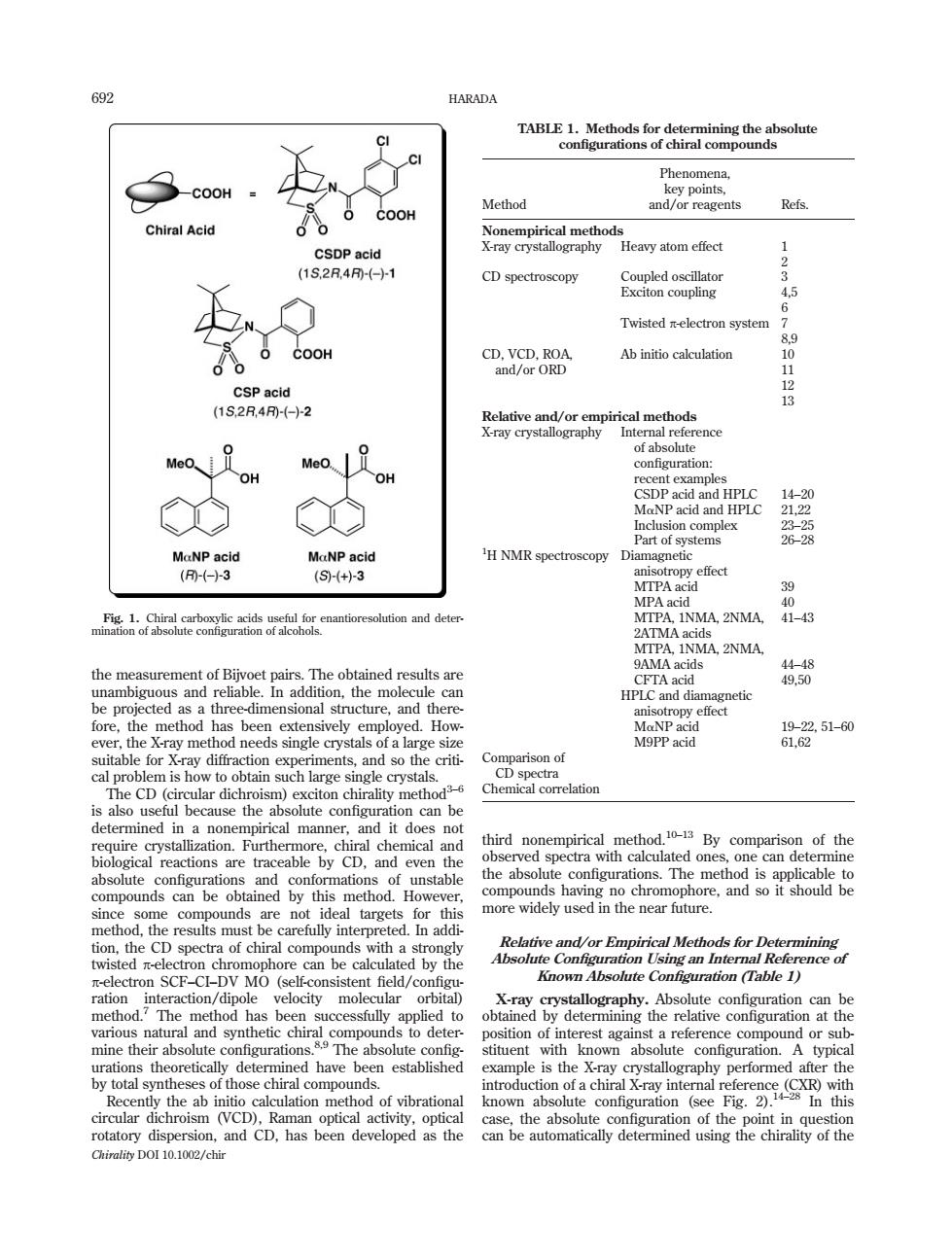
HARADA ☐N Phenomena. COOH Method and/or reagents Refs. ChiralAcid Non L Heavy atom effect 12 CD spectroscopy 5 Twisted electron system 6 CDORD Ab initio calculation (5 901213 ofahsoltio MaNP acid (-(+3 MPA acid MA,2NMA. the r 特8 How the method has been exten 品泾51-0 ab Chemical correlation ecause the abs eoagdd third n unstable this no chromophore.and so it should be thi more widely used in the near future. ted by the The obt mine their absolute urations theoretically determined have been established configuration stituent with La ch Raertaheahneakhtoanehodofbrnaional known abso configurati rotatory dispersion,and CD.has been developed as the Chirality DOI 10.1002/chir the measurement of Bijvoet pairs. The obtained results are unambiguous and reliable. In addition, the molecule can be projected as a three-dimensional structure, and therefore, the method has been extensively employed. However, the X-ray method needs single crystals of a large size suitable for X-ray diffraction experiments, and so the critical problem is how to obtain such large single crystals. The CD (circular dichroism) exciton chirality method3–6 is also useful because the absolute configuration can be determined in a nonempirical manner, and it does not require crystallization. Furthermore, chiral chemical and biological reactions are traceable by CD, and even the absolute configurations and conformations of unstable compounds can be obtained by this method. However, since some compounds are not ideal targets for this method, the results must be carefully interpreted. In addition, the CD spectra of chiral compounds with a strongly twisted p-electron chromophore can be calculated by the p-electron SCF–CI–DV MO (self-consistent field/configuration interaction/dipole velocity molecular orbital) method.7 The method has been successfully applied to various natural and synthetic chiral compounds to determine their absolute configurations.8,9 The absolute configurations theoretically determined have been established by total syntheses of those chiral compounds. Recently the ab initio calculation method of vibrational circular dichroism (VCD), Raman optical activity, optical rotatory dispersion, and CD, has been developed as the third nonempirical method.10–13 By comparison of the observed spectra with calculated ones, one can determine the absolute configurations. The method is applicable to compounds having no chromophore, and so it should be more widely used in the near future. Relative and/or Empirical Methods for Determining Absolute Configuration Using an Internal Reference of Known Absolute Configuration (Table 1) X-ray crystallography. Absolute configuration can be obtained by determining the relative configuration at the position of interest against a reference compound or substituent with known absolute configuration. A typical example is the X-ray crystallography performed after the introduction of a chiral X-ray internal reference (CXR) with known absolute configuration (see Fig. 2).14–28 In this case, the absolute configuration of the point in question can be automatically determined using the chirality of the TABLE 1. Methods for determining the absolute configurations of chiral compounds Method Phenomena, key points, and/or reagents Refs. Nonempirical methods X-ray crystallography Heavy atom effect 1 2 CD spectroscopy Coupled oscillator 3 Exciton coupling 4,5 6 Twisted p-electron system 7 8,9 CD, VCD, ROA, and/or ORD Ab initio calculation 10 11 12 13 Relative and/or empirical methods X-ray crystallography Internal reference of absolute configuration: recent examples CSDP acid and HPLC 14–20 MaNP acid and HPLC 21,22 Inclusion complex 23–25 Part of systems 26–28 1 H NMR spectroscopy Diamagnetic anisotropy effect MTPA acid 39 MPA acid 40 MTPA, 1NMA, 2NMA, 2ATMA acids 41–43 MTPA, 1NMA, 2NMA, 9AMA acids 44–48 CFTA acid 49,50 HPLC and diamagnetic anisotropy effect MaNP acid 19–22, 51–60 M9PP acid 61,62 Comparison of CD spectra Chemical correlation Fig. 1. Chiral carboxylic acids useful for enantioresolution and determination of absolute configuration of alcohols. 692 HARADA Chirality DOI 10.1002/chir