正在加载图片...
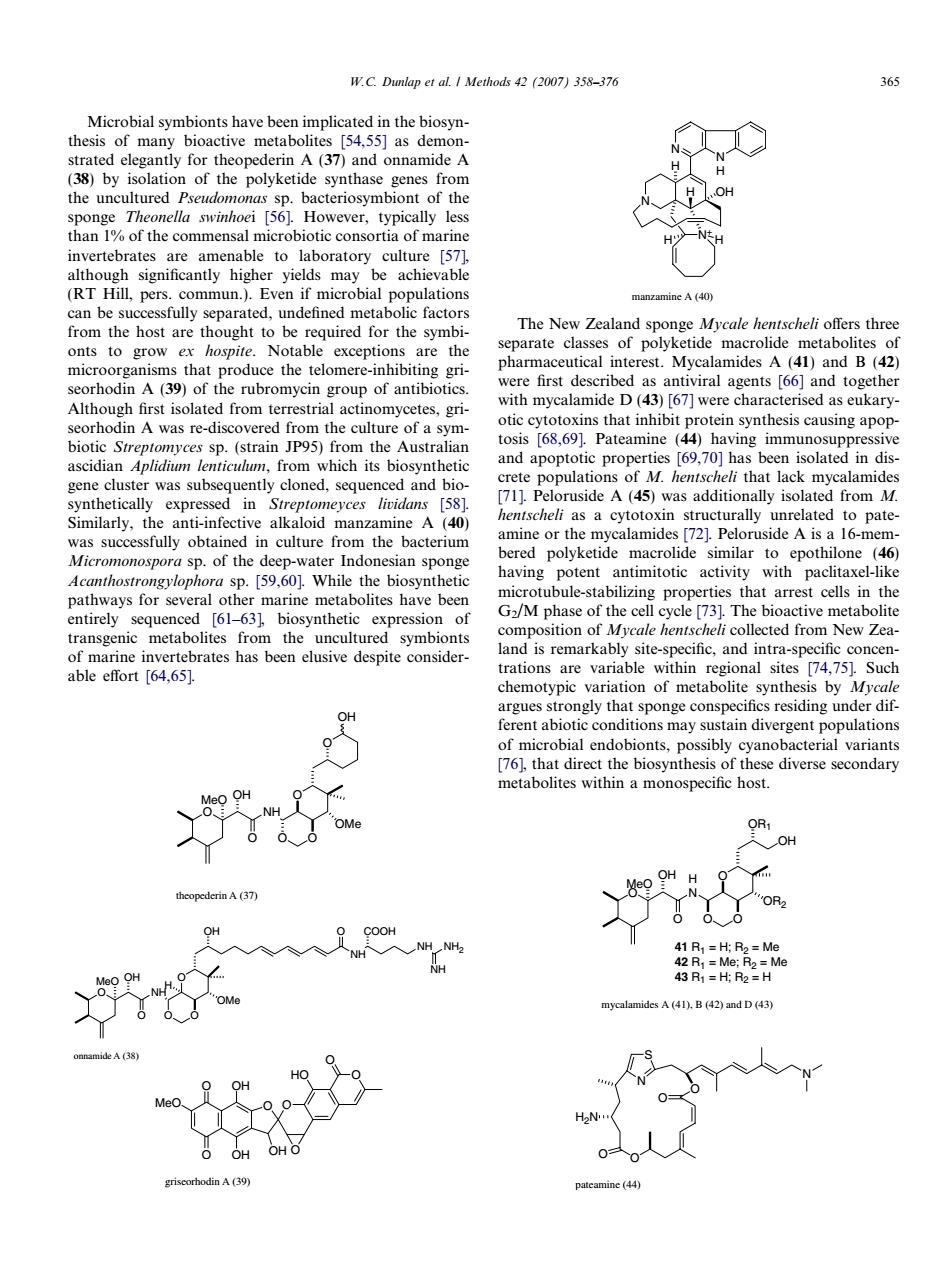
W.C.Dumlap et al.I Methods 4(2007)358-376 Microbial symbionts have been implicated in the biosyn- thesis of many bioactive metabolites [54.55]as demon- (37) and onnamide A sponge Theonella swinhoei [56].However,typically less than %of the commensal microbiotic consoria of marine sucefull ed udeamed metabie amine A(40 rom ost are ough The New Zealand sponge Mycale hentscheli offers thre eparate classes of poly d and B( first described as antiviral agents 66 and tog with mycalamide D(43)[67]were characterised as eukary n A was re vered po the cu a sym otic cytotoxins that inhibit protein synthesis causing apop having immunosuppr opulations ofM.that lack mycalamides nthetical expres th as a cytoto was successfully obtained in culture from the bacterium bered polvketide macrolide similar to epothilone (46) Acanthostrongyloph Wh having le the potent antimitotic activity with paclitaxel-like s nave bee otubule-stabilizing prope arrest cells the and is remarkably site-specific,and intra-specific concen Such of metabolit by ferent abiotic conditions may sustain divergent populations of microbial endobionts,possibly cyanobacterial variants neta OH mycalamides A(41).B(42)and D (43) 入人 人 e44) Microbial symbionts have been implicated in the biosynthesis of many bioactive metabolites [54,55] as demonstrated elegantly for theopederin A (37) and onnamide A (38) by isolation of the polyketide synthase genes from the uncultured Pseudomonas sp. bacteriosymbiont of the sponge Theonella swinhoei [56]. However, typically less than 1% of the commensal microbiotic consortia of marine invertebrates are amenable to laboratory culture [57], although significantly higher yields may be achievable (RT Hill, pers. commun.). Even if microbial populations can be successfully separated, undefined metabolic factors from the host are thought to be required for the symbionts to grow ex hospite. Notable exceptions are the microorganisms that produce the telomere-inhibiting griseorhodin A (39) of the rubromycin group of antibiotics. Although first isolated from terrestrial actinomycetes, griseorhodin A was re-discovered from the culture of a symbiotic Streptomyces sp. (strain JP95) from the Australian ascidian Aplidium lenticulum, from which its biosynthetic gene cluster was subsequently cloned, sequenced and biosynthetically expressed in Streptomeyces lividans [58]. Similarly, the anti-infective alkaloid manzamine A (40) was successfully obtained in culture from the bacterium Micromonospora sp. of the deep-water Indonesian sponge Acanthostrongylophora sp. [59,60]. While the biosynthetic pathways for several other marine metabolites have been entirely sequenced [61–63], biosynthetic expression of transgenic metabolites from the uncultured symbionts of marine invertebrates has been elusive despite considerable effort [64,65]. O NH O O O O MeO OH OMe O OH theopederin A (37) O NH O O O NH OH O NH NH2 COOH NH O OMe H MeO OH onnamide A (38) O O O MeO O O OH OH OH O O HO griseorhodin A (39) N N H N H N+ H OH H H manzamine A (40) The New Zealand sponge Mycale hentscheli offers three separate classes of polyketide macrolide metabolites of pharmaceutical interest. Mycalamides A (41) and B (42) were first described as antiviral agents [66] and together with mycalamide D (43) [67] were characterised as eukaryotic cytotoxins that inhibit protein synthesis causing apoptosis [68,69]. Pateamine (44) having immunosuppressive and apoptotic properties [69,70] has been isolated in discrete populations of M. hentscheli that lack mycalamides [71]. Peloruside A (45) was additionally isolated from M. hentscheli as a cytotoxin structurally unrelated to pateamine or the mycalamides [72]. Peloruside A is a 16-membered polyketide macrolide similar to epothilone (46) having potent antimitotic activity with paclitaxel-like microtubule-stabilizing properties that arrest cells in the G2/M phase of the cell cycle [73]. The bioactive metabolite composition of Mycale hentscheli collected from New Zealand is remarkably site-specific, and intra-specific concentrations are variable within regional sites [74,75]. Such chemotypic variation of metabolite synthesis by Mycale argues strongly that sponge conspecifics residing under different abiotic conditions may sustain divergent populations of microbial endobionts, possibly cyanobacterial variants [76], that direct the biosynthesis of these diverse secondary metabolites within a monospecific host. 41 R1 = H; R2 = Me 42 R1 = Me; R2 = Me 43 R1 = H; R2 = H O H N OOO O OR2 OH OR1 OH MeO mycalamides A (41), B (42) and D (43) N S N O O H2N O O pateamine (44) W.C. Dunlap et al. / Methods 42 (2007) 358–376 365