正在加载图片...
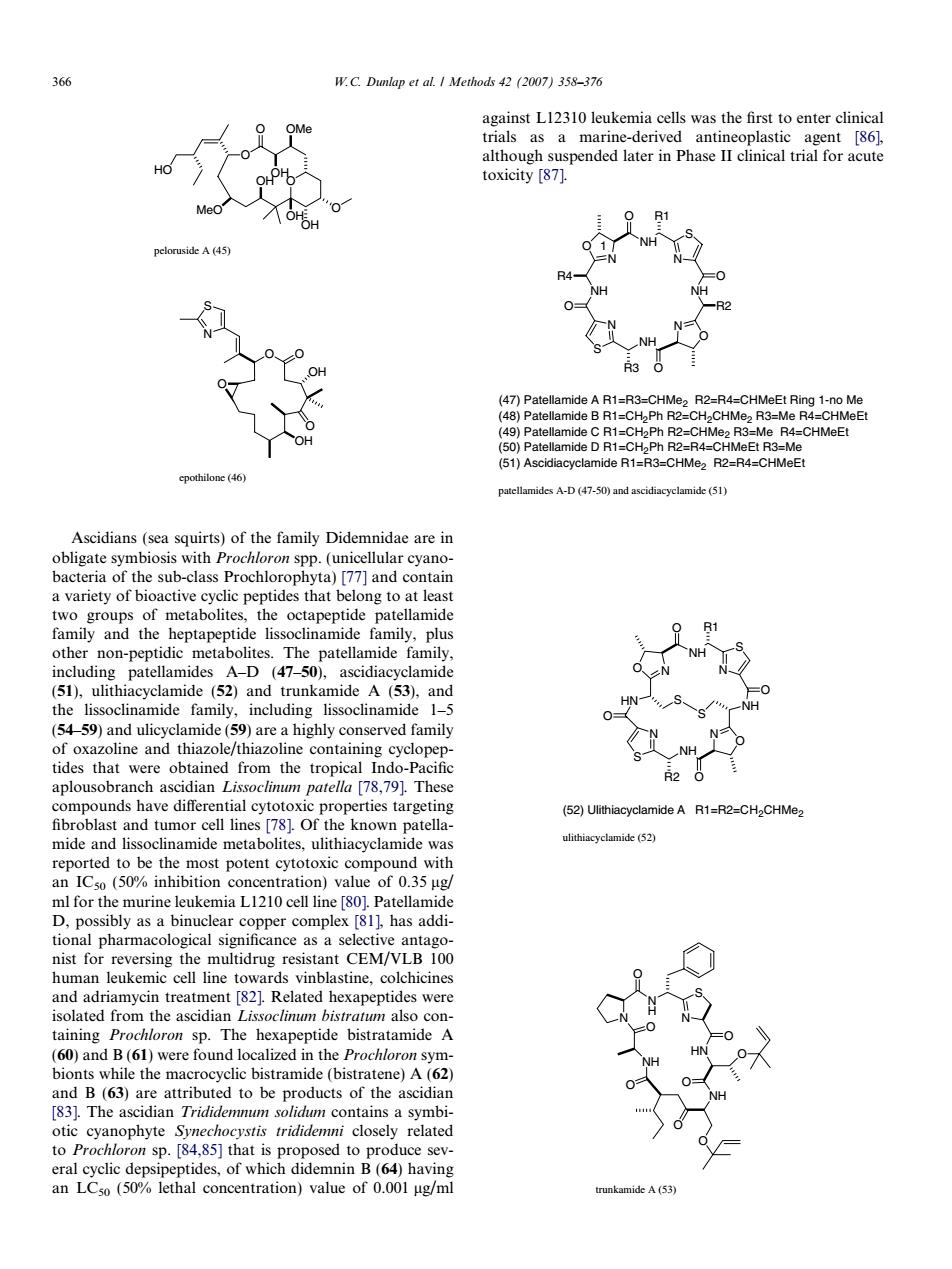
366 W.C.Dunlap et al I Methods 42 (2007)358-376 against L12310 leukemia cells was the first to enter clinical 9 0B1 NH -CHaPh R2-C 2R3- -CHMeE (51)Ascidilacyclamide R1-R3-CHMe R2-F4-CHMeE Ascidians(sea squirts)of the family Didemnidae are in teria family and the heptapeptide lissoclinamide family,plus ide N the lissoclinamide family,including lissoclinamide 1-5 HN lne and NH the (52)UlithiacyclamideA R1-R2-CH2CHMe. known patella reported to be the most potent eytotoxic compound with an ICso(50%inhibition concentration)value of 0.35 ug has nist for reversing the multidrug resistant CEM/VLB 100 human leukemic cell line towards vinblastine,colchicine treatment [82).Re s wer taining Prochloron sp.The hexapeptide bistratamide A scidian Tridid to be producis otic yanophyte Symechocystis closely related to Proc to pro ice sev les,of wh tionO OMe O O HO MeO OH OHOH O OH peloruside A (45) O OH O OH O S N O epothilone (46) Ascidians (sea squirts) of the family Didemnidae are in obligate symbiosis with Prochloron spp. (unicellular cyanobacteria of the sub-class Prochlorophyta) [77] and contain a variety of bioactive cyclic peptides that belong to at least two groups of metabolites, the octapeptide patellamide family and the heptapeptide lissoclinamide family, plus other non-peptidic metabolites. The patellamide family, including patellamides A–D (47–50), ascidiacyclamide (51), ulithiacyclamide (52) and trunkamide A (53), and the lissoclinamide family, including lissoclinamide 1–5 (54–59) and ulicyclamide (59) are a highly conserved family of oxazoline and thiazole/thiazoline containing cyclopeptides that were obtained from the tropical Indo-Pacific aplousobranch ascidian Lissoclinum patella [78,79]. These compounds have differential cytotoxic properties targeting fibroblast and tumor cell lines [78]. Of the known patellamide and lissoclinamide metabolites, ulithiacyclamide was reported to be the most potent cytotoxic compound with an IC50 (50% inhibition concentration) value of 0.35 lg/ ml for the murine leukemia L1210 cell line [80]. Patellamide D, possibly as a binuclear copper complex [81], has additional pharmacological significance as a selective antagonist for reversing the multidrug resistant CEM/VLB 100 human leukemic cell line towards vinblastine, colchicines and adriamycin treatment [82]. Related hexapeptides were isolated from the ascidian Lissoclinum bistratum also containing Prochloron sp. The hexapeptide bistratamide A (60) and B (61) were found localized in the Prochloron symbionts while the macrocyclic bistramide (bistratene) A (62) and B (63) are attributed to be products of the ascidian [83]. The ascidian Trididemnum solidum contains a symbiotic cyanophyte Synechocystis trididemni closely related to Prochloron sp. [84,85] that is proposed to produce several cyclic depsipeptides, of which didemnin B (64) having an LC50 (50% lethal concentration) value of 0.001 lg/ml against L12310 leukemia cells was the first to enter clinical trials as a marine-derived antineoplastic agent [86], although suspended later in Phase II clinical trial for acute toxicity [87]. NH S O N N NH O N NH S N NH R1 O R3 O O R2 R4 O (47) Patellamide A R1=R3=CHMe2 R2=R4=CHMeEt Ring 1-no Me (48) Patellamide B R1=CH2Ph R2=CH2CHMe2 R3=Me R4=CHMeEt (49) Patellamide C R1=CH2Ph R2=CHMe2 R3=Me R4=CHMeEt (50) Patellamide D R1=CH2Ph R2=R4=CHMeEt R3=Me (51) Ascidiacyclamide R1=R3=CHMe2 R2=R4=CHMeEt 1 patellamides A-D (47-50) and ascidiacyclamide (51) NH O N N S R2 O HN O N O NH N S O R1 O NH S S (52) Ulithiacyclamide A R1=R2=CH2CHMe2 ulithiacyclamide (52) N N H O N S HN O O NH O O NH O O O trunkamide A (53) 366 W.C. Dunlap et al. / Methods 42 (2007) 358–376