正在加载图片...
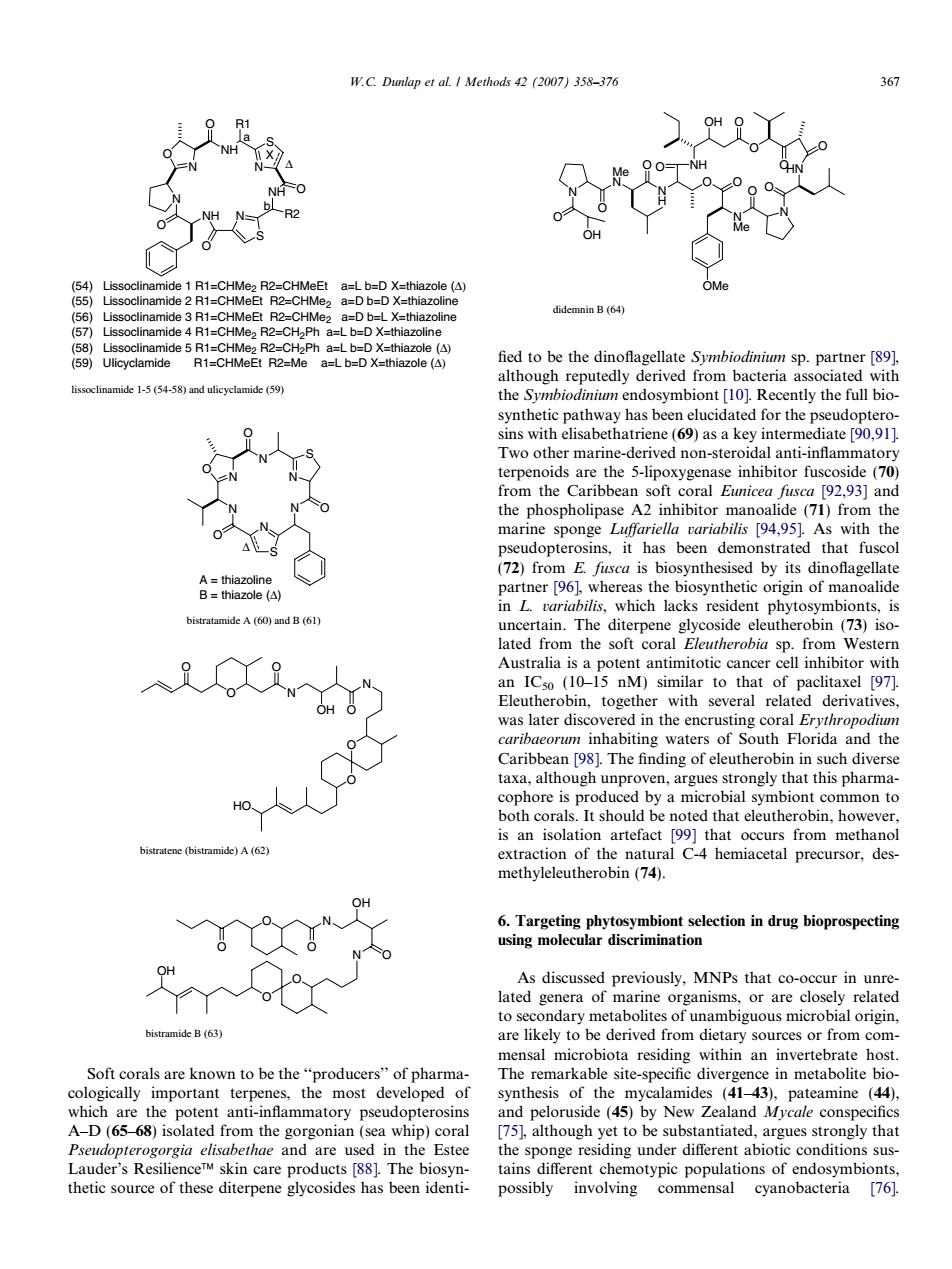
W.C.Dumlap et al.I Methods 4(2007)358-370 R1-CHMOERCMCE a=L D=D X( aB(64 fied to be the dinoflagellate Symbiodinium sp.partner [89]. mide 1-5 (54-58)and ulicyelamide alhotehcpucdtyndcrnve atrine (ine Tpohcrmainetdcednonsteroidalantinnammao r0203 0 marine sponge Luffariella rariabilis 94.95 As with the ins,it has bee hat fusco agea inL tariabilis,which lacks resident phytosymbionts,is an IC (10-15 nM)similar to that of paclitaxel 97 Eleutherobin,together with several related derivatives was later disc hin i ch dive taxa,although unproven,argues strongly that this pharma 99)tha eleuthe extraction of the natural C4 hemiacetal precursor,des- methyleleutherobin(74). to secondary metabolites of unambiguous istramide B(63) are likely to be derived from dietary sources or from com nensal microbiota residing within an host te-spe amides (4t-43) (44 which are the potent anti-inflammatory pseudopterosins nd Peloruside (5 Newald Myclo A-D(6568)isolated from the gorgonian (sea whip)cora thae and are the Estee thetic source of these diterpene glycosides has been identi- possibly involving commensal cyanobacteria 76] NH S O N N N NH O S N NH O R2 O R1 O X (54) Lissoclinamide 1 R1=CHMe2 R2=CHMeEt a=L b=D X=thiazole (Δ) (55) Lissoclinamide 2 R1=CHMeEt R2=CHMe2 a=D b=D X=thiazoline (56) Lissoclinamide 3 R1=CHMeEt R2=CHMe2 a=D b=L X=thiazoline (57) Lissoclinamide 4 R1=CHMe2 R2=CH2Ph a=L b=D X=thiazoline (58) Lissoclinamide 5 R1=CHMe2 R2=CH2Ph a=L b=D X=thiazole (Δ) (59) Ulicyclamide R1=CHMeEt R2=Me a=L b=D X=thiazole (Δ) a b Δ lissoclinamide 1-5 (54-58) and ulicyclamide (59) N N S O N N N O S N O O Δ A = thiazoline B = thiazole (Δ) bistratamide A (60) and B (61) O N N O O HO OH O O O bistratene (bistramide) A (62) O N N O O O O OH O OH bistramide B (63) Soft corals are known to be the ‘‘producers’’ of pharmacologically important terpenes, the most developed of which are the potent anti-inflammatory pseudopterosins A–D (65–68) isolated from the gorgonian (sea whip) coral Pseudopterogorgia elisabethae and are used in the Estee Lauder’s Resilience skin care products [88]. The biosynthetic source of these diterpene glycosides has been identi- fied to be the dinoflagellate Symbiodinium sp. partner [89], although reputedly derived from bacteria associated with the Symbiodinium endosymbiont [10]. Recently the full biosynthetic pathway has been elucidated for the pseudopterosins with elisabethatriene (69) as a key intermediate [90,91]. Two other marine-derived non-steroidal anti-inflammatory terpenoids are the 5-lipoxygenase inhibitor fuscoside (70) from the Caribbean soft coral Eunicea fusca [92,93] and the phospholipase A2 inhibitor manoalide (71) from the marine sponge Luffariella variabilis [94,95]. As with the pseudopterosins, it has been demonstrated that fuscol (72) from E. fusca is biosynthesised by its dinoflagellate partner [96], whereas the biosynthetic origin of manoalide in L. variabilis, which lacks resident phytosymbionts, is uncertain. The diterpene glycoside eleutherobin (73) isolated from the soft coral Eleutherobia sp. from Western Australia is a potent antimitotic cancer cell inhibitor with an IC50 (10–15 nM) similar to that of paclitaxel [97]. Eleutherobin, together with several related derivatives, was later discovered in the encrusting coral Erythropodium caribaeorum inhabiting waters of South Florida and the Caribbean [98]. The finding of eleutherobin in such diverse taxa, although unproven, argues strongly that this pharmacophore is produced by a microbial symbiont common to both corals. It should be noted that eleutherobin, however, is an isolation artefact [99] that occurs from methanol extraction of the natural C-4 hemiacetal precursor, desmethyleleutherobin (74). 6. Targeting phytosymbiont selection in drug bioprospecting using molecular discrimination As discussed previously, MNPs that co-occur in unrelated genera of marine organisms, or are closely related to secondary metabolites of unambiguous microbial origin, are likely to be derived from dietary sources or from commensal microbiota residing within an invertebrate host. The remarkable site-specific divergence in metabolite biosynthesis of the mycalamides (41–43), pateamine (44), and peloruside (45) by New Zealand Mycale conspecifics [75], although yet to be substantiated, argues strongly that the sponge residing under different abiotic conditions sustains different chemotypic populations of endosymbionts, possibly involving commensal cyanobacteria [76]. NH OH O O O O HN O N O N Me OMe O O O N H O Me N O N O OH didemnin B (64) W.C. Dunlap et al. / Methods 42 (2007) 358–376 367