正在加载图片...
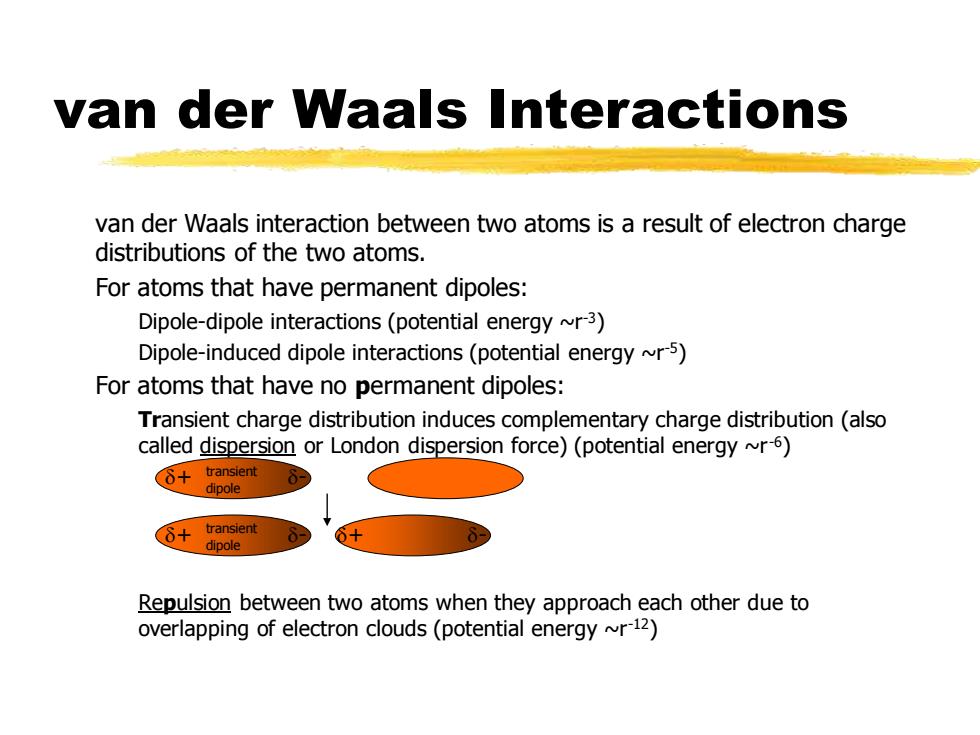
van der Waals Interactions van der Waals interaction between two atoms is a result of electron charge distributions of the two atoms. For atoms that have permanent dipoles: Dipole-dipole interactions(potential energy ~r3) Dipole-induced dipole interactions(potential energy r5) For atoms that have no permanent dipoles: Transient charge distribution induces complementary charge distribution(also called dispersion or London dispersion force)(potential energy ~r 6) (心+transient dipole 6+ transient dipole Repulsion between two atoms when they approach each other due to overlapping of electron clouds(potential energy ~r12)van der Waals Interactions van der Waals interaction between two atoms is a result of electron charge distributions of the two atoms. For atoms that have permanent dipoles: Dipole-dipole interactions (potential energy ~r-3) Dipole-induced dipole interactions (potential energy ~r-5) For atoms that have no permanent dipoles: Transient charge distribution induces complementary charge distribution (also called dispersion or London dispersion force) (potential energy ~r-6) Repulsion between two atoms when they approach each other due to overlapping of electron clouds (potential energy ~r-12) d+ d- d+ dd+ dtransient dipole transient dipole