正在加载图片...
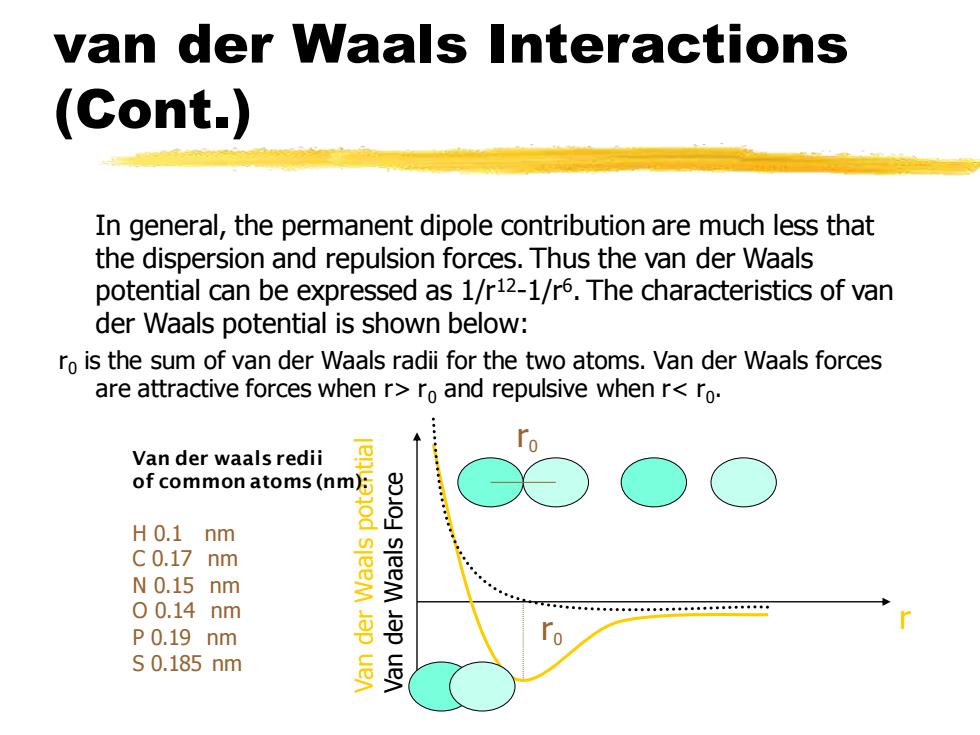
van der Waals Interactions (Cont.) In general,the permanent dipole contribution are much less that the dispersion and repulsion forces.Thus the van der Waals potential can be expressed as 1/r12-1/r6.The characteristics of van der Waals potential is shown below: ro is the sum of van der Waals radii for the two atoms.Van der Waals forces are attractive forces when r>ro and repulsive when r<ro. Van der waals redii of common atoms(nm): H0.1 nm C0.17 nm N0.15 nm sleeM 00.14nm P0.19 nm S0.185nm sleeM Jap uen van der Waals Interactions (Cont.) In general, the permanent dipole contribution are much less that the dispersion and repulsion forces. Thus the van der Waals potential can be expressed as 1/r12-1/r6 . The characteristics of van der Waals potential is shown below: r0 is the sum of van der Waals radii for the two atoms. Van der Waals forces are attractive forces when r> r0 and repulsive when r< r0 . r Van der Waals potential Van der Waals Force r0 r0 Van der waals redii of common atoms (nm): H 0.1 nm C 0.17 nm N 0.15 nm O 0.14 nm P 0.19 nm S 0.185 nm