正在加载图片...
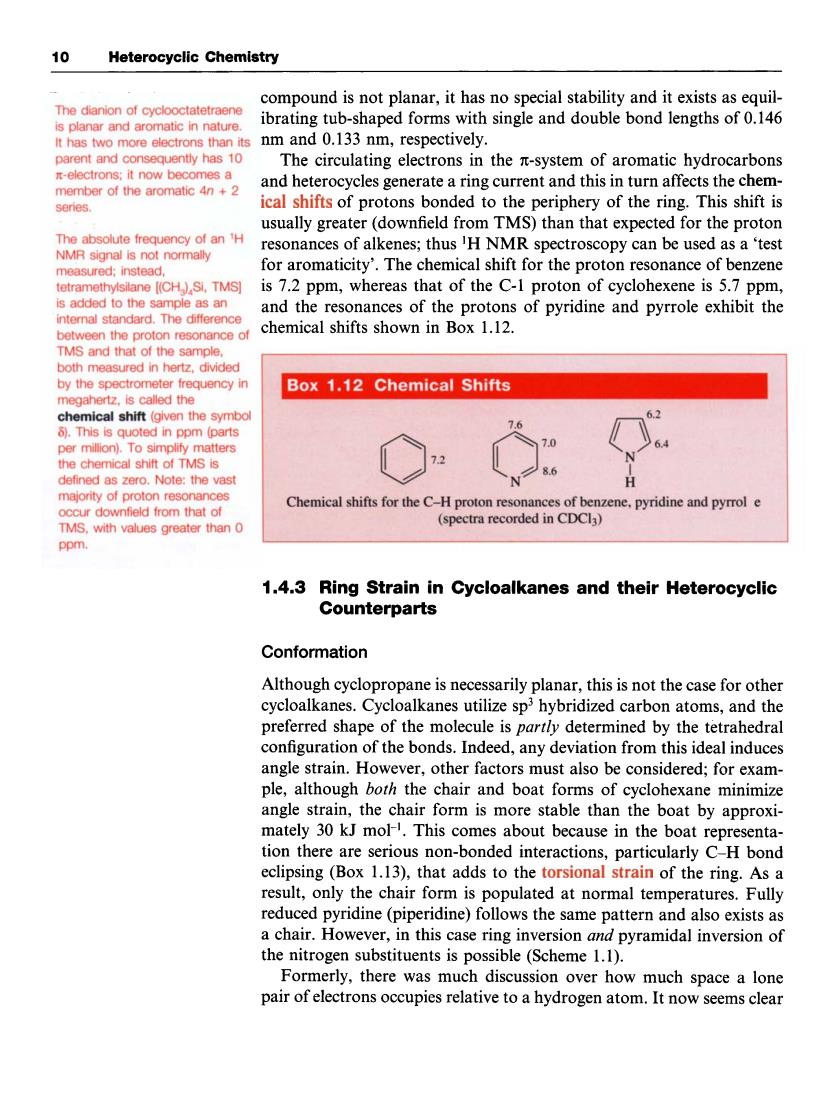
0 Heterocyclic Chemistry compound is not planar,it has no special stability and it exists as equil- The dianion of cyck ibrating tub-shaped forms with single and double bond lengths of 0.146 Ithas two more electrons than its nm and 0.133 nm,respectively. parent and cons equently has 10 The circulating electrons in the n-system of aromatic hydrocarbons atic 4n+2 and heterocycles generate a ring current and this in turn affects the chem- series. icl shifts of protons bonded to h periphery of the ring.This shift is usually greater(downfield from TMS)than that expected for the proton resonances of alkenes;thus'H NMR spectroscopy can be used as a'test measured:instead. for aromaticity'.The chemical shift for the proton resonance of benzene is pm,whereas that of of. ntemal standard The difference and the resonances of the protons of pyridine and pyrrole exhibit the chemical shifts shown in Box 1.12. ed in h sa by the spectrometer frequency in Box 1.12 Chemical Shifts is called the d in pom 1.4.3 Ring Strain in Cycloalkanes and their Heterocyclic Counterparts Conformation Although cyclopropane is ecessarily planar,this is not the case for other cycloalkanes.Cycloalkanes utilize sphybridized carbon atoms,and the preferred shape of the molecule is partly determined by the tetrahedral configuration of the bonds.Indeed,any deviation from this ideal induces angle strain.However,other factors must also be considered;for exam ple,although both the chair and boat forms of cyclohexane minimize angle strain,the chair form is more stable than the boat by approxi- mately 30 kJ mol-.This comes about because in the boat representa- tion there are serious non-bonded interactions, eclipsing(Box 1.13),that adds to the torsional strain of the ring.As a result,only the chair form is populated at normal temperatures.Fully reduced pyridine (piperidine)follows the same pattern and also exists as a chair.However,in this case ring inversion and pyramidal inversion of the nitrog en sube stituents is possible(Scheme 1.1). Formerly,there was much discussion over how much space a lone pair of electrons occupies relative to a hydrogen atom.It now seems clear 10 Heterocyclic Chemistry The dianion of cyclooctatetraene is planar and aromatic in nature. It has two more electrons than its parent and consequently has 10 x-electrons; it now becomes a member of the aromatic 4n + 2 series. The absolute frequency of an 'H NMR signal is not normally measured; instead, tetramethylsilane [(CH,),Si, TMS] is added to the sample as an internal standard. The difference between the proton resonance of TMS and that of the sample, both measured in hertz, divided by the spectrometer frequency in megahertz, is called the chemical shift (given the symbol 8). This is quoted in ppm (parts per million). To simplify matters the chemical shift of TMS is defined as zero. Note: the vast majority of proton resonances occur downfield from that of TMS, with values greater than 0 PPm. compound is not planar, it has no special stability and it exists as equilibrating tub-shaped forms with single and double bond lengths of 0.146 nm and 0.133 nm, respectively. The circulating electrons in the n-system of aromatic hydrocarbons and heterocycles generate a ring current and this in turn affects the chemical shifts of protons bonded to the periphery of the ring. This shift is usually greater (downfield from TMS) than that expected for the proton resonances of alkenes; thus 'H NMR spectroscopy can be used as a 'test for aromaticity'. The chemical shift for the proton resonance of benzene is 7.2 ppm, whereas that of the C-1 proton of cyclohexene is 5.7 ppm, and the resonances of the protons of pyridine and pyrrole exhibit the chemical shifts shown in Box 1.12. I .4.3 Ring Strain in Cycloalkanes and their Heterocyclic Counterparts Conformation Although cyclopropane is necessarily planar, this is not the case for other cycloalkanes. Cycloalkanes utilize sp3 hybridized carbon atoms, and the preferred shape of the molecule is partly determined by the tetrahedral configuration of the bonds. Indeed, any deviation from this ideal induces angle strain. However, other factors must also be considered; for example, although both the chair and boat forms of cyclohexane minimize angle strain, the chair form is more stable than the boat by approximately 30 kJ mol-I. This comes about because in the boat representation there are serious non-bonded interactions, particularly C-H bond eclipsing (Box 1.13), that adds to the torsional strain of the ring. As a result, only the chair form is populated at normal temperatures. Fully reduced pyridine (piperidine) follows the same pattern and also exists as a chair. However, in this case ring inversion and pyramidal inversion of the nitrogen substituents is possible (Scheme 1.1). Formerly, there was much discussion over how much space a lone pair of electrons occupies relative to a hydrogen atom. It now seems clear