正在加载图片...
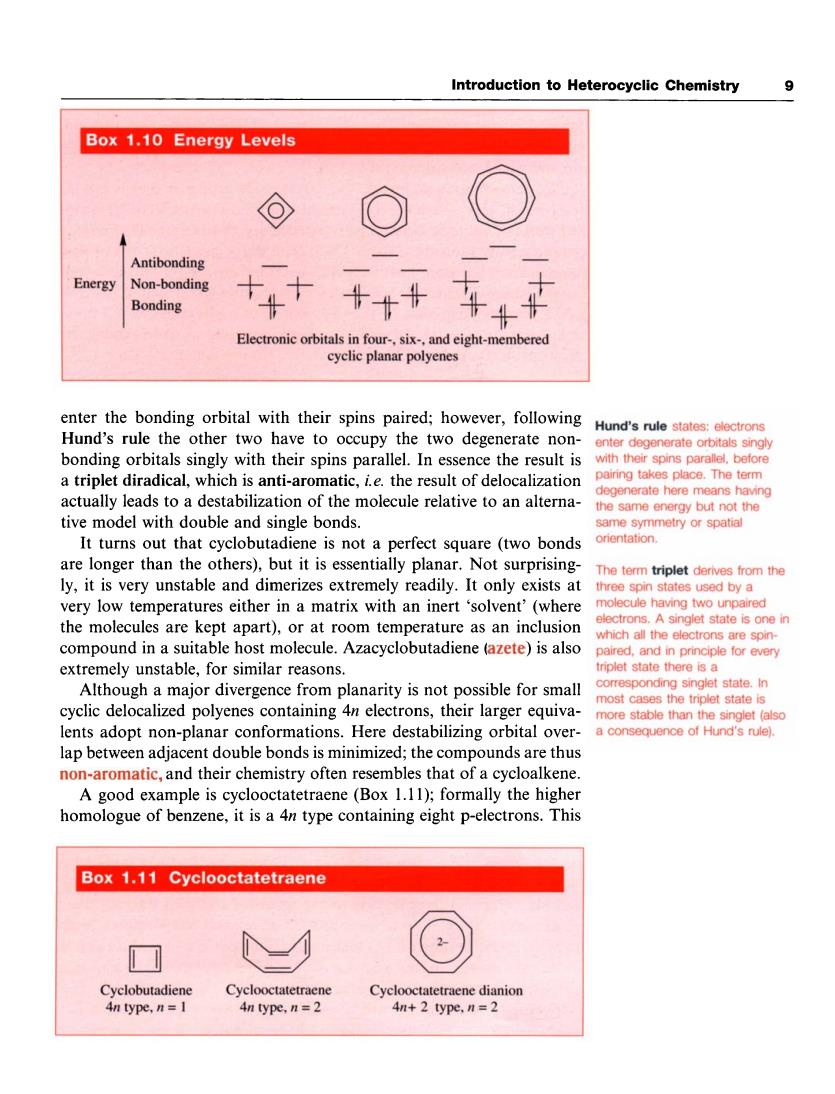
Introduction to Heterocyclic Chemistry 9 Box 1.10 Energy Levels ⊙ ( Antibonding Energy Non-bonding Bonding +++ 车4中 Electronic orbitals in four-.six-,and eight-membered cyclic planar polyenes enter the bonding orbital with their spins paired:however,following Hund's rule the other two have to occupy the two degenerate non- Hund's rule states:electrons enter degenerate orbitals singly bonding orbitals singly with their spins parallel.In essence the result is a triplet diradical,which is anti-aromatic,ie.the result of delocalization actually leads to a destabilization of the molecule relative to an alterna- enerate here means having tive model with double and sir gle bonds the same energy but not the It turns out that cyclobutadiene is not a perfect square (two bonds are longer than the others),but it is essentially planar.Not surprising- ly,it is very unstable and dimerizes extremely readily.It only exists at ery low temperatures either in a matrix with an inert 'solvent'(where ecule having two ur ed the molecules are kept apart),or at room temperature as an inclusion electrons.A singlet state is one in compound in a suitable host molecule.Azacyclobutadiene (azete)is also extremely unstable,for similar reasons. triplet state there is a Alth ugh a major divergence from planarity is not possible for small cyclic delocalized polyenes containing 4n electrons,their larger equiva- more stable than the singlet (alsd lents adopt non-planar conformations.Here destabilizing orbital over- a consequence of Hund's rule). lap between adjacent double bonds is minimized;the compounds are thus -aromatic,and their chemistry often resembles that of a cycloalkene A good example is cyclooctatetraene(Box 1.11);formally the higher homologue of benzene,it is a 4n type containing eight p-electrons.This Box 1.11 Cyclooctatetraene Cyclobutadien Cyclooctatetra Cyclooctatetraene dianion type,n= 4n+2 type,n=2Introduction to Heterocyclic Chemistry 9 enter the bonding orbital with their spins paired; however, following Hund’s rule the other two have to occupy the two degenerate nonbonding orbitals singly with their spins parallel. In essence the result is a triplet diradical, which is anti-aromatic, i. e. the result of delocalization actually leads to a destabilization of the molecule relative to an alternative model with double and single bonds. It turns out that cyclobutadiene is not a perfect square (two bonds are longer than the others), but it is essentially planar. Not surprisingly, it is very unstable and dimerizes extremely readily. It only exists at very low temperatures either in a matrix with an inert ‘solvent’ (where the molecules are kept apart), or at room temperature as an inclusion compound in a suitable host molecule. Azacyclobutadiene (azete) is also extremely unstable, for similar reasons. Although a major divergence from planarity is not possible for small cyclic delocalized polyenes containing 4n electrons, their larger equivalents adopt non-planar conformations. Here destabilizing orbital overlap between adjacent double bonds is minimized; the compounds are thus non-aromatic, and their chemistry often resembles that of a cycloalkene. A good example is cyclooctatetraene (Box 1.1 1); formally the higher homologue of benzene, it is a 4n type containing eight p-electrons. This Hund’s rule states: electrons enter degenerate orbitals singly with their spins parallel, before pairing takes place. The term degenerate here means having the same energy but not the same symmetry or spatial orientation. The term triplet derives from the three spin states used by a molecule having two unpaired electrons. A singlet state is one in which all the electrons are spinpaired, and in principle for every triplet state there is a corresponding singlet state. In most cases the triplet state is more stable than the singlet (also a consequence of Hund’s rule)