正在加载图片...
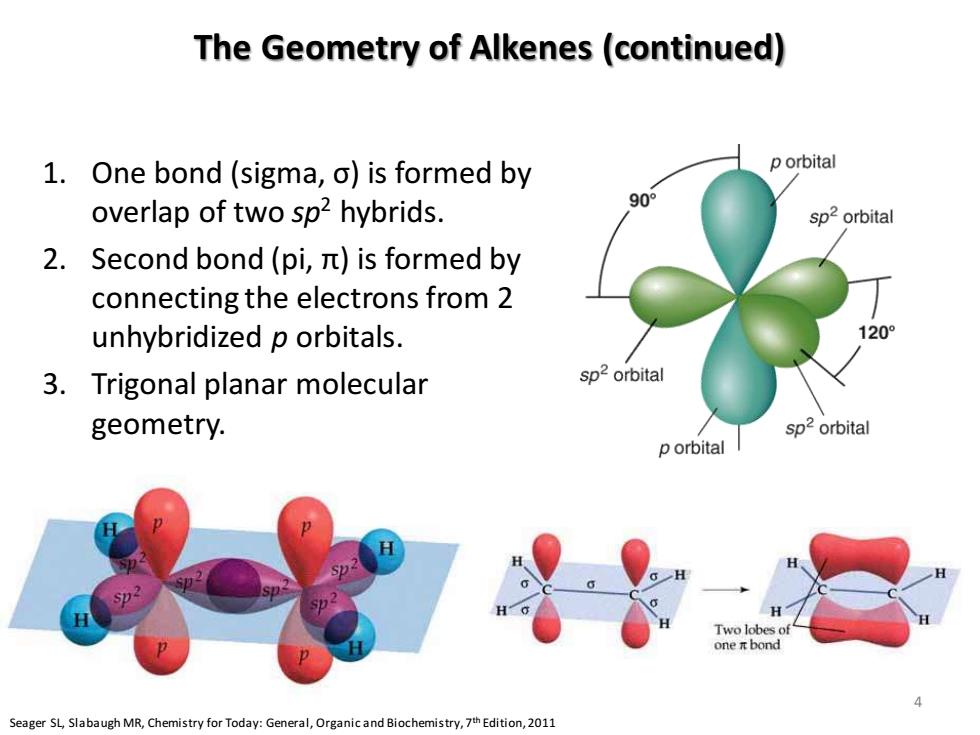
The Geometry of Alkenes(continued) 1.One bond (sigma,o)is formed by p orbital overlap of two sp2 hybrids. 90° sp2orbital 2.Second bond(pi,it)is formed by connecting the electrons from 2 unhybridized p orbitals. 1209 3.Trigonal planar molecular sp2 orbital geometry. sp2 orbital p orbital l H Two lobes of one i bond Seager SL,Slabaugh MR,Chemistry for Today:General,Organic and Biochemistry,7th Edition,2011 The Geometry of Alkenes (continued) 1. One bond (sigma, σ) is formed by overlap of two sp2 hybrids. 2. Second bond (pi, π) is formed by connecting the electrons from 2 unhybridized p orbitals. 3. Trigonal planar molecular geometry. 2p 2s 1s Energy 2p 3 sp2 1s Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 4