正在加载图片...
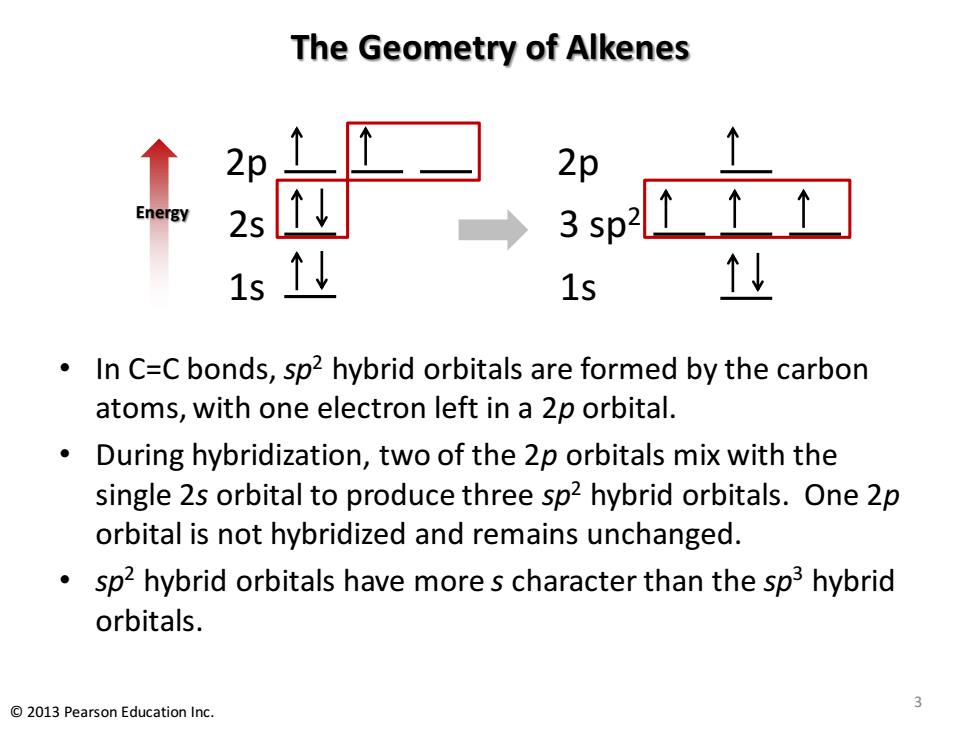
The Geometry of Alkenes 2p 2p Energy 2s 3sp2↑↑↑ 15 1s 型 In C=C bonds,sp2 hybrid orbitals are formed by the carbon atoms,with one electron left in a 2p orbital. During hybridization,two of the 2p orbitals mix with the single 2s orbital to produce three sp2 hybrid orbitals.One 2p orbital is not hybridized and remains unchanged. sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. 3 2013 Pearson Education Inc.The Geometry of Alkenes • In C=C bonds, sp2 hybrid orbitals are formed by the carbon atoms, with one electron left in a 2p orbital. • During hybridization, two of the 2p orbitals mix with the single 2s orbital to produce three sp2 hybrid orbitals. One 2p orbital is not hybridized and remains unchanged. • sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. 2p 2s 1s Energy 2p 3 sp2 1s 3 © 2013 Pearson Education Inc